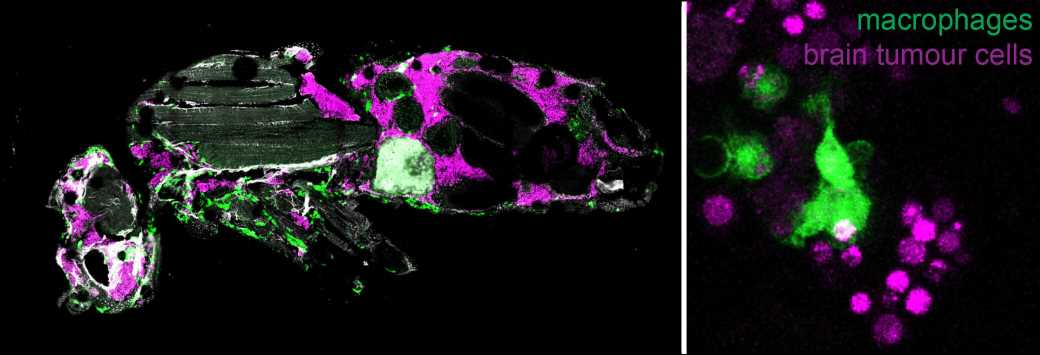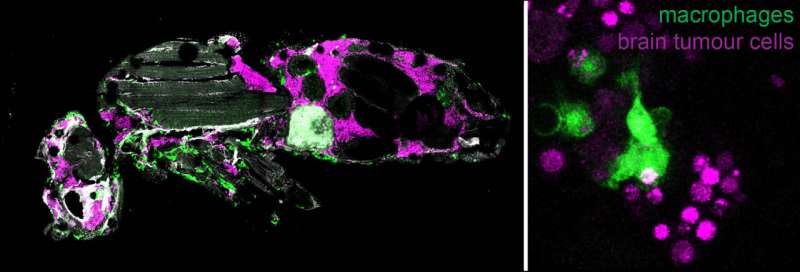
A new research study at the Institute of Molecular Biology and Biotechnology (IMBB) of the Foundation for Research and Technology-Hellas (FORTH), published in the journal PNAS, reveals how cancer cell intrinsic mechanisms and interactions with the microenvironment shape brain tumor growth and progression to malignancy.
Every year, six out of 100,000 people (children and adults) are diagnosed with brain, and other nervous system, cancer. Most of these patients will undergo therapeutic strategies combining surgery with radio- and chemotherapy. However, only 33% of these patients will survive for more than five years. The life expectancy for the majority will be circa 18 months to two years.
Although there is a great research interest in neural tumors, current therapeutic strategies still remain ineffective. One of the reasons why brain tumors are hard to cure is because they are comprised of diverse cell types, both cancerous and normal.
Only a subpopulation of these cells carry the combination of mutations that contributes to tumor emergence and maintenance. These cells are called cancer stem cells and it is thought that, to achieve tumor growth, they hijack developmental programs that, under physiological conditions, neural stem cells use to generate diverse cells types of neurons and glia during development and upon tissue repair.
The scientific community believes that brain tumor progression to a malignant state is the result of an intricate interplay between deregulated intrinsic mechanisms in some cancer stem cells and interactions with the tumor microenvironment, including cells of the immune system that infiltrate tumor masses.
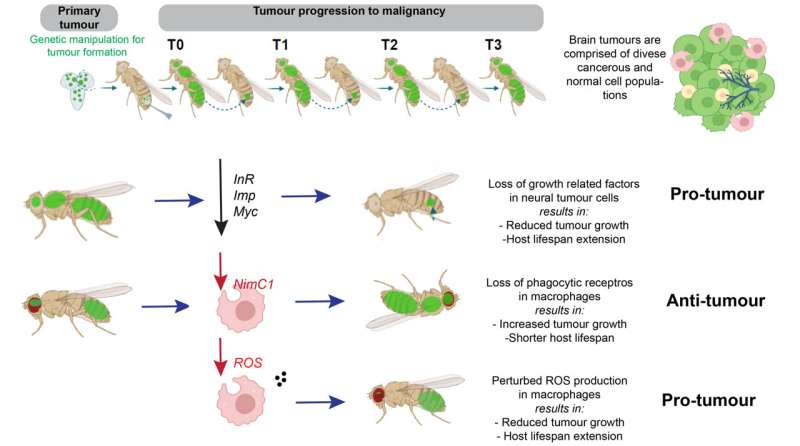
In order to study mechanisms of tumourigenesis and malignancy in the brain, IMBB researchers Dr. Chrysanthi Voutyraki, Dr. Evanthia Zacharioudaki and Dr. Christos Delidakis, (Professor at the Biology Department of the University of Crete), used the fruit fly D. melanogaster where, via a simple genetic manipulation, they generated a neural stem cell derived tumor.
They discovered that, as tumors progress to malignancy, developmental processes related to neuronal differentiation are shut down, whereas processes related to growth, metabolism and immunity are upregulated.
By using sophisticated genetic tools, they showed that two pathways related to growth, more specifically the oncogene Myc and the Insulin receptor are necessary for tumor progression to malignancy. They also observed that macrophages from the host animals (the frontline soldiers of the body in the defense against invading pathogens) infiltrate tumors and resist/impede tumor growth inside their host.
Live imaging revealed that macrophages engulf and consume tumor cells (a process known as phagocytosis) thus delaying tumor growth. When macrophages lack surface receptors involved in the phagocytosis of pathogens (like NimC1), they can no longer trap and engulf tumor cells thus tumors grow faster and kill their host earlier. Finally, while phagocytosis resists tumor expansion, the researchers discovered that macrophages also promote tumor growth by their natural propensity to produce reactive oxygen species (ROS) which contributes to faster host demise.
These findings contribute to a better understanding of neural tumor growth strategies and their complex interactions with their microenvironment.
In the future, these Insights could serve as pointers for routes to follow in studies using mammalian systems. In the long run, they could enable the design and development of novel and effective chemical or immunological approaches to target specifically the proliferation of cancer stem cells in brain related tumors (e.g glioblastoma) thus improving the life expectancy of brain cancer patients.
More information:
Chrysanthi Voutyraki et al, Growth deregulation and interaction with host hemocytes contribute to tumor progression in a Drosophila brain tumor model, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2221601120
Journal information:
Proceedings of the National Academy of Sciences
Source: Read Full Article