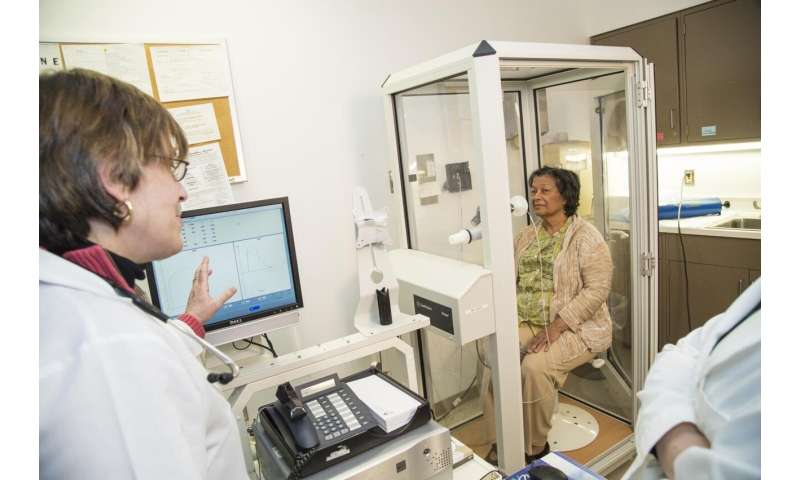
An American Thoracic Society-led international task force has released a guidance document to help guide clinicians on restoring elective in-person pulmonary and sleep services as COVID-19 incidence decreases in their communities. The new guidance, published online in the Annals of the American Thoracic Society, is titled “Restoring Pulmonary and Sleep Services as the COVID-19 Pandemic Lessens.”
The task force, comprised of clinicians who are members of the Association of Pulmonary, Critical Care, and Sleep Division Directors and/or the American Thoracic Society who are actively engaged in COVID-19 patient care, developed a consensus approach on how and when to restart services that were put on hold due to the Centers for Disease Control and Prevention (CDC) March 2020 recommendations to cancel elective services. Specific guidance is provided regarding elective services in outpatient pulmonary and sleep medicine clinics, pulmonary function testing laboratories, bronchoscopy and procedural suites, polysomnography laboratories, and pulmonary rehabilitation facilities.
“This document provides important guidance to health care institutions about when it is reasonable to begin resuming elective in-person clinical services in pulmonary and sleep medicine, as well as strategies to mitigate the risk of viral transmission as those services are resumed,” said Kevin C. Wilson, MD, chief of Guidelines and Documents at the American Thoracic Society and Professor of Medicine at Boston University School of Medicine. “To facilitate implementation of the guidance, we aimed to account for limitations in staff, equipment and space that are essential for the care of COVID-19 patients and provide access to care for patients with acute and chronic conditions.”
The main recommendations for resuming outpatient clinical services are:
- to ensure that the local new case rate has a downward trajectory for at least 14 days before resuming clinical testing, assuming that the volume of testing remains relatively constant;
- to resume elective clinical services when one’s institution has the capacity for implementing patient prioritization, screening, diagnostic testing, physical distancing, infection control and follow-up surveillance;
- to prioritize outpatient services on the basis of patient acuity, and tailor services to institutional resources, patient and provider preferences, and community disease prevalence;
- to identify patients with SARS-CoV-2, the virus caused by COVID-19, by following a multi-phased screening schedule to mitigate the possibility of viral transmission from such patients;
- to use physical distancing strategies, which should vary depending on COVID-19 community prevalence, and should also account for visitor policies;
- to institute appropriate infection control and personal protective equipment protocols, such as requiring that all patients wear a surgical mask and cleaning rooms between patients;
- to instruct patients to contact the clinic if they develop new respiratory symptoms within 14 days of their visit, and/or are diagnosed with COVID-19;
- to, periodically, critically assess the success (or lack thereof) of resuming pulmonary and sleep medicine clinical services and adjust accordingly, and
- to give staff either COVID-19 or non-COVID assignments, with no rotations through both clinical settings.
Guidance for specific services include, for example, evaluating how important pulmonary function testing is for making a diagnosis or decision, relative to the risk of exposing staff and cross-contaminating equipment. For bronchoscopy and procedure suites, a priority scoring system such as the Medically Necessary and Time-Sensitive instrument might be used to determine procedure scheduling. The guidance recommends that polysomnography services reopen in a phased manner, to allow staff time to acclimate to a new workflow, with the preferred first step being home sleep apnea testing.
Source: Read Full Article
