Together with colleagues, veterinarian and neuroscientist Sonja Bröer has researched how regenerative cell therapies can contribute to curing or alleviating epilepsy. The work was carried out at biotechnology start-up Neurona Therapeutics, Inc. in San Francisco, where Bröer led a team in preclinical research, before she moved to Freie Universität Berlin.
The company is developing a cell therapy (NRTX-1001) for treatment-resistant epilepsy and has now published the results from preclinical studies in Cell Stem Cell. Based on these data, the cell therapy is now being evaluated in human patients as part of an ongoing phase 1/2 clinical trial. On October 6, 2023, Bröer will present both the preclinical and the first clinical data at the Einstein Center for Neurosciences’ Berlin Neuroscience Meeting.
Approximately 50 million people worldwide suffer from epilepsy; in about one-third of patients, epileptic seizures do not respond to drug treatment, reducing patients’ quality of life as well as their life expectancy. Epilepsy occurs when excessive electrical discharges occur in nerve cells of the brain.
The neurotransmitter gamma-aminobutyric acid (GABA) can block this overexcitation. However, the nerve cells that secrete this neurotransmitter can degenerate in patients with epilepsy, creating an imbalance between inhibition and excitation in the brain that is thought to pave the way for epileptic seizure activity.
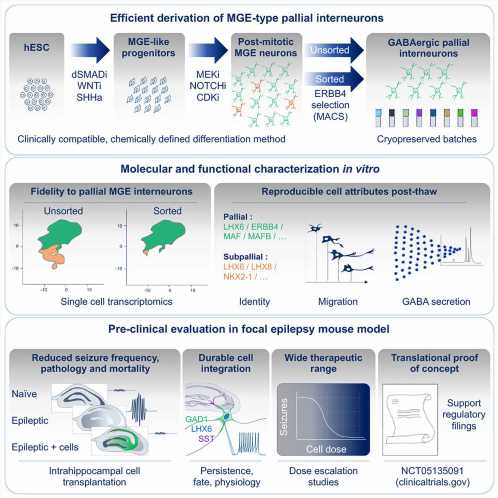
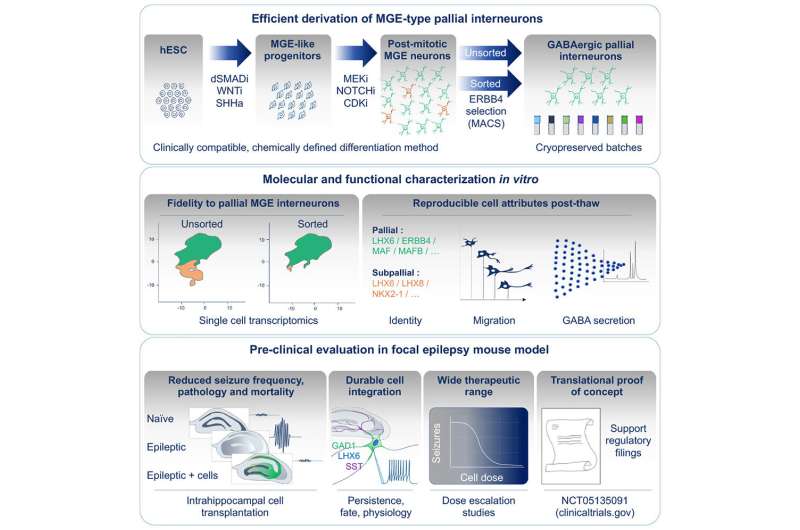
In their recent publication in Cell Stem Cell, Bröer and her colleagues report on the transplantation of inhibitory GABA-secreting neurons that can potentially restore the balance in the brain and suppress epileptic seizures. Developing and characterizing such a cell therapy could provide a breakthrough alternative for patients with treatment-resistant seizures.
Developed by Neurona Therapeutics, cell therapy NRTX-1001 is derived from a human embryonic stem cell line and differentiated into inhibitory neurons. Administering NRTX-1001 into a mouse model of chronic epilepsy resulted in long-lasting and reproducible seizure suppression, with most animals becoming completely seizure-free.
The transplanted inhibitory neurons distributed throughout the brain and integrated themselves into the host’s existing neuronal network. The cells survived long-term and also reduced other pathological side effects of epilepsy, such as scarring in the brain, which also occurs in human patients.
These disease-modifying effects were dose-dependent. No adverse effects were observed. These results support an ongoing phase 1/2 clinical trial (NCT05135091) for drug-resistant epilepsy. If approved, this would be the first cell therapy for epilepsy worldwide and an alternative that could transform the lives of millions of patients suffering from treatment-resistant seizures.
More information:
Marina Bershteyn et al, Human pallial MGE-type GABAergic interneuron cell therapy for chronic focal epilepsy, Cell Stem Cell (2023). DOI: 10.1016/j.stem.2023.08.013
Journal information:
Cell Stem Cell
Source: Read Full Article