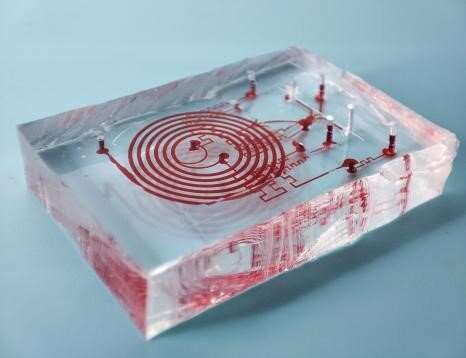
A paper by scientists at the Hangzhou Dianzi University proposed a 3D-stacked multi-stage inertial microfluidic sort chip to enrich and separate Circulating Tumor Cells (CTCs). The new paper, published in the journal Cyborg and Bionic Systems, provided a multi-stage integration of the spiral and serpentine channels that can meet the requirements of flow rate input for high-throughput and flow rate output for downstream detection.
“Circulating tumor cells (CTCs) found in peripheral blood, showing a wealth of information are proven to be a marker of cancer diagnosis and treatment monitoring,” says senior author Huang, a professor at Hangzhou Dianzi University. “However, reliable isolation of viable and intact CTCs is often hard to achieve due to the extremely low numbers of rare cells in peripheral blood (only 1-100CTC/ml) and massive background cells.”
Numerous microfluidic platforms have been developed in the past decades to isolate target cells for the need of biomedical research and clinical diagnosis.
“Compared with the traditional large-volume cell sorting technology applied in clinic at this stage, the cell separation technology based on microfluidic can accurately manipulate the displacement of liquid or cells on the micro-scale. Due to the small size of equipment, low cost, and less sample consumption, it has great potential and high operation accuracy in the application of point of care testing (POCT),” said study author Xu.
The separation and detection of biological samples can often rely on their physical properties such as size, deformability, mass, electrical properties, etc. Since CTCs have an apparent size difference from other cells such as red blood cells (RBCs) or white blood cells (WBCs), they can be separated by size.
Inertial microfluidics is one of the label-free techniques for the enrichment of tumor cells in high throughput and passive manners. The balance between the inertial lift force and Dean drag force leads to a well-defined equilibrium position of the particles or cells at a certain flow rate in spiral channels, based on their size.
The newly proposed 3D-stacked multi-stage inertial microfluidic cell sorting chip integrated a trapezoidal spiral channel and two square serpentine channels. The trapezoidal spiral channel is the first stage of the chip with one inlet and two outlets. The channels of different stages were arranged from top to bottom in line with the liquid flow direction with only one inlet for the whole chip without sheath flow.
Moreover, to guarantee that all stages of channels could work in optimal working states, matching channels were also designed to match the flow resistance.
“After automatic multi-stage removal of RBCs and the deceleration, aggregation, and concentration of target flow, the flow rate was reduced from mL/min at the inlet to μL/min at the outlet, which made it easier for integration with downstream detection and analysis such as impedance detection and imaging analysis,” said Xu.
To characterize the performance of this chip, the tumor cells were to be detected, the results show that it can separate tumor cells (SW480, A549, and Caki-1) from a large number of RBCs with a separation efficiency of >80%, separation purity of 90%, and a concentration fold of ~20.
“Towards practical application, high flow rate input for high-throughput and low flow rate output for easy downstream detection are usually simultaneously required,” said Huang, the designed 3D-stacked multi-stage microfluidic chip overcomes the limitation between separation purity, separation efficiency, and throughput in a single-stage chip, and provides a promising basis for integrated downstream detection methods through multi-stage flow rate reduction.
Source: Read Full Article
