Researchers have developed a comprehensive database of drugs that could be repurposed to tackle mutated SARS-CoV-2 viruses, narrowing the search from thousands to a select few candidates. The research is published in the journal Data.
Although vaccines have proved effective against the virus and its variants, they are not able to prevent their transmission, and so new drugs are being sought to keep pace with the continuously mutating disease.
One approach to this challenge is to use drugs already in-use for other conditions, but research has shown that there are more than 7,800 compounds that could potentially fit the criteria, and narrowing down this list would be essential in order to move forward with any measure of success.
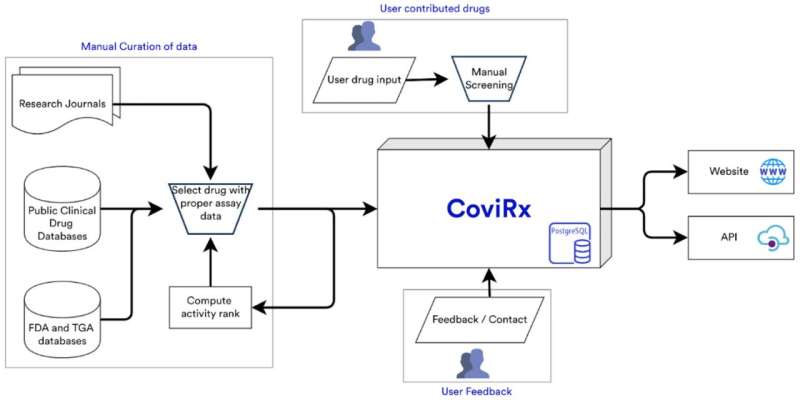
A consortium of researchers, led by Honorary Professor Seshadri Vasan from the University of York’s Department of Health Sciences, however, has now released a database of drugs that allows scientists to narrow their search from a list of 7,817 potential candidates to a “top 200.” Of these, 12 have been tested experimentally at the Australian Centre for Disease Preparedness, and a few show sufficient promise for further investigation.
Professor Vasan said, “We have an urgent and critical need to identify antiviral drugs that are safe, effective and affordable. Drug repurposing is one strategy to explore, but with nearly eight thousand compounds to choose from, it can seem like an endless ‘treasure hunt’ of possibilities.
“With this new database, we can’t say that we have found the exact drug that we need, but we have made the hunt for it considerably easier, with 12 drugs tested and awaiting further investigation, some 200 more candidates for the global research community to evaluate, and the potential for this resource to be used for other diseases too.”
The database, called CoviRx, presents the properties of each drug, as well as data from COVID-19 experiments and clinical trials, any “red flags,” and similar drugs in use.
Source: Read Full Article
