Researchers at Weill Cornell Medicine have identified a key protein that induces the program to build specialized liver blood vessels. The discovery could lead to engineered replacement hepatic tissue to treat common liver diseases.
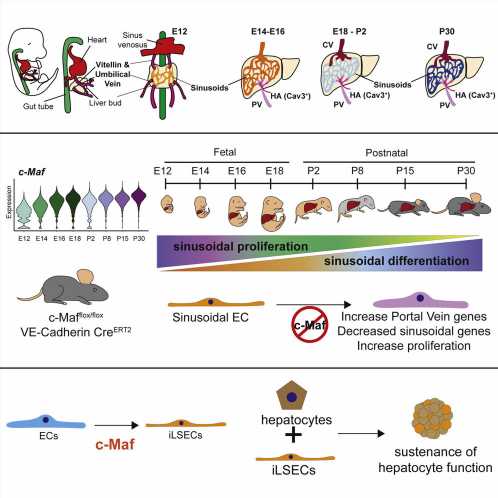
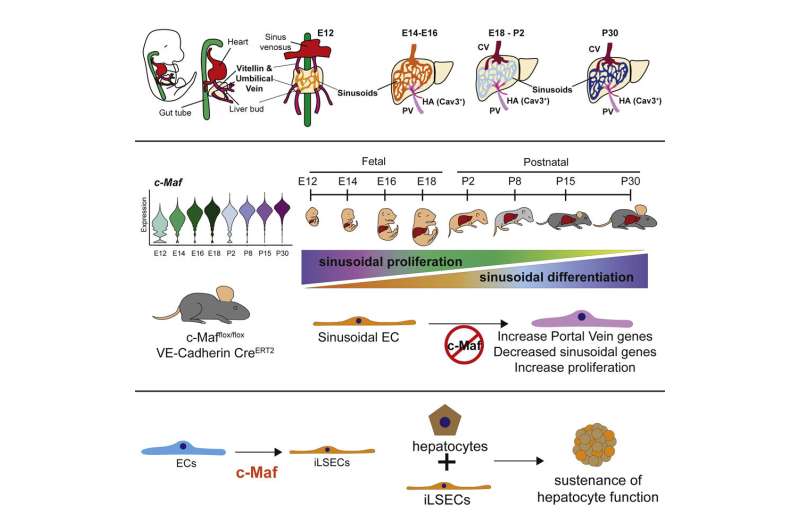
There are many types of blood vessels in the human body that are functionally different from each other. In the liver, these vessels are organized into distinct zones marked by “zip codes,” which are designated by the expression of specific proteins. Sinusoidal endothelial cells form one of the specialized liver small blood vessel types, that through crosstalk with hepatocytes—the main cell type in the liver—orchestrate the crucial tasks of controlling inflammatory and immune responses, detoxifying chemicals, and producing key proteins for blood coagulation. These unique blood vessels also secrete growth factors that drive liver regeneration, thus protecting the liver from damage and injury, for example from excessive alcohol use, viral hepatitis or a high-fat diet.
In the study, published March 31 in Cell Stem Cell, the researchers found that a protein called c-Maf—a member of the transcription factor class of proteins that control large sets of genes—is required for naive endothelial cells to mature specifically into liver sinusoidal blood vessels. The researchers used the protein to create sinusoidal cells from other human endothelial cells in the lab and showed that these induced sinusoidal cells can help sustain the health of hepatocytes.
“These discoveries will set the stage for pre-clinical trials in which permanently damaged blood vessels could be replaced with healthy, engineered liver sinusoidal cells to expedite regeneration and repair,” said study co-senior author Dr. Shahin Rafii, chief of the Division of Regenerative Medicine, director of the Ansary Stem Cell Institute and the Arthur B. Belfer Professor in Genetic Medicine at Weill Cornell Medicine. The newly established Hartman Institute for Therapeutic Organ Regeneration, housed within Dr. Rafii’s division, will be key to further exploring the study’s medical implications.
Chronic liver diseases affect at least several million American adults and cause about 50,000 deaths per year, according to the U.S. Centers for Disease Control and Prevention. Among the most common liver diseases are alcohol-related liver cirrhosis, nonalcoholic fatty liver disease, and hepatitis B and C virus infections. All involve an inflammatory process that results in fibrosis, in which healthy tissue is progressively damaged and replaced by fibrotic, scar-like tissue, impairing the liver’s functions.
“Advanced liver disease and fibrosis currently is incurable except with a liver transplant, an expensive and surgically challenging option that not all patients are candidates for and often cannot be arranged in time, due to the shortage of suitable donor livers,” said co-senior author Dr. Robert Schwartz, associate professor of medicine in the Division of Gastroenterology and Hepatology at Weill Cornell Medicine. “Therefore, there is an unmet need to develop new therapeutics, such as building mini-livers to treat these end-stage maladies.”
Prior studies have suggested that sinusoidal and other vessel-lining endothelial cells in the liver can help the organ resist fibrosis and even partly regenerate itself after injury. That in turn has suggested the possibility of making these cells artificially to use as cell therapies for liver disorders. However, to do that, biologists would need to learn much more about how these cells develop naturally in the liver.
In this new study, the researchers tracked gene activity in tens of thousands of individual endothelial cells in the livers of embryonic and newborn mice, showing how distinct types of liver endothelial cell emerge from immature progenitor cells.
“This comprehensive analysis revealed the transcription factor c-Maf as a critical switch controlling sinusoidal endothelial cell maturation and specification into highly unique blood vessels that are customized to support the daily demands and function of hepatocytes,” said study first author Dr. Jesus Maria Gómez-Salinero, an instructor of medicine in the Rafii laboratory. “When we deleted c-Maf specifically in endothelial cells, this resulted in disrupted development of sinusoidal blood vessels rendering mouse livers more vulnerable to fibrosis after damage.”
By contrast, forcing the human version of c-Maf to become active in unspecialized human endothelial cells, caused these cells to develop the distinctive markers of liver sinusoidal endothelial cells. The researchers termed these cells “induced liver sinusoidal endothelial cells” (iLSECs). Leveraging sophisticated bioinformatic analyses by a group of computational biologists, Drs. Franco Izzo, Dan Landau, and David Redmond at Weill Cornell Medicine, revealed that iLSECs have acquired the majority of the hallmarks of the supportive native liver sinusoidal blood vessels. Remarkably, these iLSECs support hepatocytes, keeping them healthy and functioning over long periods when they would otherwise perish in culture dishes.
Source: Read Full Article