Scientists at Texas Biomedical Research Institute (Texas Biomed) and Southwest National Primate Research Center (SNPRC) published their findings regarding a comprehensive animal model study of SARS-CoV-2 in the peer-reviewed journal Nature Microbiology. These findings were originally posted online in BioRxiv in June of 2020. The study evaluated three nonhuman primate (NHP) species (Indian rhesus macaques, African baboons and new-world origin common marmosets) and young and old animals, to determine susceptibility to the SARS-CoV-2 virus and the development of COVID-19 disease. Over the course of the study, the macaque and baboon models showed significant promise as animal models for COVID-19 disease studies moving forward. Based on outcomes, the researchers recommended use of the macaque as a model to help develop vaccines, while the baboon showed greater disease development, making it a potential option for evaluating anti-viral therapeutics and co-morbidities, such as understanding the connection between COVID-19 and diabetes or COVID-19 and heart disease.
“Thanks to the support of our community, Texas Biomed was able to launch and complete the most comprehensive animal model study to date (June 2020) that has provides scientists greater understanding of the immune response to SARS-CoV-2 and definitively identifies two possible animal models to help move vaccines and therapeutics forward,” said Larry Schlesinger, M.D., Texas Biomed President and CEO. “The speed and completeness to which this team of researchers operated to execute this study is nothing short of heroic. I am very pleased at the progress the Institute is making in our COVID-19 studies and believe this is the first of many discoveries to come.”
COVID-19 is the defining pandemic of a generation. Texas Biomed launched this study in late March 2020 because animal models are a critical component of the biomedical pipeline necessary to fast track drug and vaccine development. The study was completed by May 2020.
“The benefit of Texas Biomed’s unique research model lies in the expertise to support individual scientific study and contract research on one campus with the animal, biosafety and regulatory proficiency to help shepherd research from basic discovery through preclinical development and on to human clinical trials,” explained Joanne Turner, Ph.D., Vice President for Research at Texas Biomed.
Animal models for infectious diseases, such as COVID-19, are allowing scientists worldwide to determine whether the candidate vaccines and antiviral therapeutics currently under development will be viable as human interventions. Additionally, animal models enable scientists to understand how the disease progresses in people with compromised immune systems to assist in the development of treatments for these individuals.
“Finding the appropriate animal models for COVID-19 allows for these critical discoveries to happen now, and they are an important step in combatting this disease,” said Dr. Deepak Kaushal, Ph.D., Director of the Southwest National Primate Research Center and lead Principal Investigator on the macaque portion of the study. “Without well-documented animal data, the FDA is unlikely to license a vaccine or antiviral therapy for human use, even those currently undergoing human trials, because animal model data assure us that we have a complete picture of the disease and how humans may respond to potential therapies.”
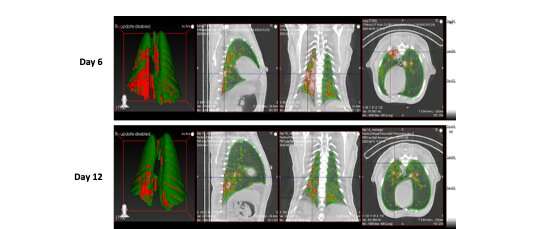
The team of 43 researchers reported clinical, viral, imaging, immunological and histopathological (tissue examination) findings during SARS-CoV-2 infection/COVID-19 disease in all three species of NHPs. The study ultimately found that nonhuman primates showed similar progression of SARS-CoV-2 infection to that of humans, with some becoming more ill than others, and signs of the virus in both the upper and lower respiratory tracts and signs of pneumonia.
“Our results tell us that these animal models will provide relevant, quantifiable information moving forward as we delve deeper into understanding the disease and targets for therapeutics and vaccines for human trials,” Dr. Kaushal explained.
While previous animal studies showed the macaque to be a viable model for SARS-CoV-2, this was the first time that researchers performed a longitudinal study of three different NHPs (looking at disease progression factors over several days) and in both young and older macaques to determine if age is a factor in disease progression. Moreover, the researchers used the most comprehensive set of evaluations, ranging from bronchoalveolar lavages (lung fluid collection) and nasal swabs to determine virus presence to chest x-rays and CT scans to evaluate lung health after infection.
Results showed that the macaque and baboon models develop strong signs of acute viral infection leading to pneumonia, and the NHP immune system mounts a strong response and clears the infection. Specialized sets of myeloid cells (phagocytes) move from blood to the lungs and secrete high levels of Type I interferons, cytokines or proteins that send chemical messages required for controlling viruses in general and coronaviruses in particular. The appearance of these specialized phagocytes (cells that ingest foreign particles or dying cells in the body) corresponded with a decline in measurable amounts of virus and disease parameters. The longitudinal study of young and old animals showed little difference. However, the virus appears to persist and shed longer in the baboons and create greater pathology in the lungs. The marmoset model did not show any significant signs of disease progression.
This study was also the first report of SARS-CoV-2 infection specifically altering lymphoid cells (T cells) in the lung, which generated a strong and very specific immune response in the macaque, enabling the animals to clear the virus. This finding indicates the NHP model will be useful in understanding the immune response to SARS-CoV-2 and aid in the development of interventions that can create a similar response, as well as help evaluate the safety and effectiveness of vaccines, which require a specific immune response in order to be effective.
“While this study was not the first to indicate the macaque would serve as a good model for SARS-CoV-2, the study provides strong scientific evidence in support of this model, as well as the first evidence of the baboon model via comprehensive, clinically-relevant and well-documented research with controls,” Dr. Kaushal said. “We strongly believe macaques and baboons will be very helpful in evaluating interventions that generate the strong immune response seen in these animal models.”
The study was funded by philanthropic support from more than 300 donors after a call-to-action campaign was launched in late March. The campaign raised more than $3.5 million in one week, and additional donor support has brought the total raise for COVID-19 research at Texas Biomed to more than $5.7 million.
Source: Read Full Article
