Introducing medical devices—commonly made of materials such as titanium, silicone, or collagen—into our bodies can elicit a host of different immune responses. While some responses can harm our bodies, others can help heal them. Researchers have not fully grasped the rhyme or reason behind the body’s reactions, but a new study fills in a critical piece of the puzzle.
By closely examining the immune system’s polarized responses to two different materials implanted in mice, researchers at the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have identified key driving factors for implant-induced regeneration and damage. The authors suggest that their findings, published Oct. 23 in Nature Materials, help set the stage for the design of new medical devices that influence the immune system to help rather than harm the body.
Medical devices such as pacemakers, breast implants, or knee replacements can trigger hostile immune responses that can damage not only the implants themselves but the surrounding tissue as well. The clinical go-to solution has been to administer drugs that tamp down the body’s immune response, known as immunosuppressants, which are not always effective and come with serious risks.
Due to complications including immune-mediated damage, additional surgeries are almost always required, be it months or years later, to remove or replace implants that have been rendered ineffective or dangerous.
However, some implants, such as naturally derived tissue grafts, have shown that they can steer the immune system in a more desirable direction, stimulating reparative processes. While natural biomaterials are not suited to every medical application, they may still hold valuable lessons for other kinds of devices.
“If we could tease out what aspect of these different materials causes certain immune responses, we would have more control in designing materials and devices that encourage the responses we want,” said senior author Kaitlyn Sadtler, Ph.D., Earl Stadtman Tenure-Track Investigator and chief of the Section on Immunoengineering at NIBIB.
To find explanations for the varying reactions to different devices, the authors of the new study removed volumes of muscle in the legs of mice and filled in the gaps with two biomaterials known to elicit opposite reactions.
One was decellularized small intestinal submucosa—pig tissue that has been stripped of its cells, leaving behind mostly collagen—which encourages reparative immune processes and has shown promise as a clinical solution for abdominal injuries. The other was polyethylene, a plastic used to make orthopedic implants that can trigger long-term inflammation and scarring as small pieces of it are shed into surrounding tissue. A third group of injured mice were left untreated for comparison.
Through a process called flow cytometry, Sadtler and her co-authors identified and counted various cell types from tissue retrieved from the mice.
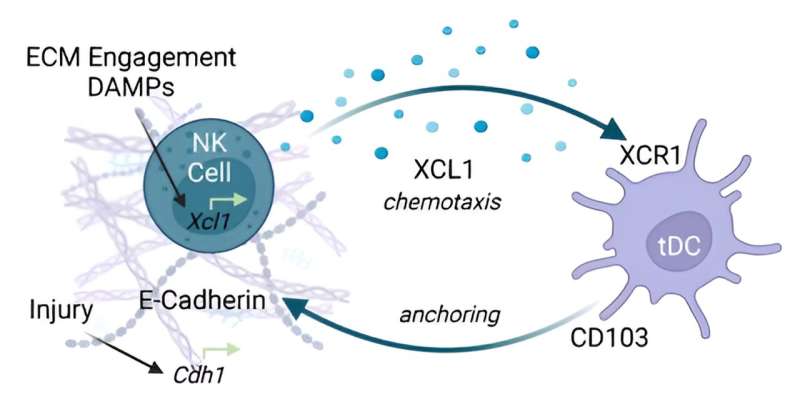
As expected, the samples implanted with submucosa displayed patterns more closely associated with anti-inflammation and repair than those implanted with polyethylene did. But, of the many differences between the samples implanted with the two types of materials, the amount of one cell type present was particularly striking.
The submucosa samples contained far more of a specific cell type called cDC1s, belonging to a class known as dendritic cells, which help the immune system tell friend from foe.
What’s more, when the researchers used mice that were genetically altered to stymie the development of the dendritic cell subtype, inflammation and scarring was rampant and the growth of new muscle was altered, regardless of the material implanted.
“These dendritic cells are likely playing a key role in calming the immune system. So, when we saw they were enriched in the samples with porcine submucosa, we wanted to dig deeper to understand how and why these cells were in the injury site,” Sadtler said.
Through a group of experiments, the researchers determined that an immune cell type called natural killer cells were secreting chemical signals to draw in the subtype of dendritic cells—a well-documented process seen in immune responses to tumors.
In the context of cancer, natural killer cells are activated by molecular danger signals released by injured or dying cells. These signals would also likely be present in pig submucosa, but not polyethylene, making these molecules a probable driver for the drastic difference in how the two groups of animals responded, Sadtler explained.
After identifying some preliminary mechanisms by which the material induces this cell recruitment, Sadtler’s team plans to identify the specific proteins and molecules that kicked off the reparative responses, with the goal of harnessing those molecules to dampen autoimmunity and amplify regenerative processes.
If their strategies bear fruit, then they could be useful for modifying existing devices—adding protein-filled coatings to the surface of a pacemaker, for example—or informing the design of entirely new devices. By playing nice with the body’s complex immune system, medical devices of the future could be safer and last much longer.
“Implants frequently cause pain and, in many cases, need to be removed within a few years. We’re looking to eventually eliminate that grief by extending the lifespan of implanted medical devices to the lifespan of patients,” Sadtler said.
More information:
Ravi Lokwani et al, Pro-regenerative biomaterials recruit immunoregulatory dendritic cells after traumatic injury, Nature Materials (2023). DOI: 10.1038/s41563-023-01689-9
Journal information:
Nature Materials
Source: Read Full Article