An astounding 30% of Americans currently have metabolic dysfunction-associated steatotic liver disease, or MASLD, which is formerly known as nonalcoholic fatty liver disease, or NAFLD, and many are unaware that they have it.
Over time, MASLD can lead to cirrhosis, liver failure and even liver cancer. The disease is also associated with and might be caused by factors that contribute to obesity, diabetes, high cholesterol and heart disease.
“Many people are not aware that MASLD can also be a sign of another condition, like diabetes or cardiovascular disease,” said Elizabeth Speliotes, M.D., Ph.D., M.P.H., gastroenterologist at Michigan Medicine and professor of internal medicine and computational medicine and bioinformatics at the University of Michigan Medical School.
“Whether an individual will eventually develop MASLD and if they do, whether they will go on to develop a more advanced liver disease or other metabolic disease is not known. Furthermore, because MASLD and these other metabolic diseases have multiple causes that we could not previously separate, current treatments are often imprecise, ineffective and/or riddled with many side effects.”
This is why Speliotes and a team of experts sought to identify the human genetic causes of MASLD.
“We wanted to better predict those individuals at highest risk for developing the disease, as well as provide direction for new therapeutics,” she said.
Their findings were recently published in Nature Genetics.
Speliotes, who is the senior author of the study, and her team combined data across four multiethnic cohorts: the Genetics of Obesity-related Liver Disease, or GOLD, Consortium with CT-measured hepatic steatosis; U.K. Biobank participants with hepatic steatosis defined by MRI diagnosis code-defined cases of MASLD from the International Classification of Diseases, or ICD; the eMERGE database, which relies on electronic medical records to define MASLD cases and controls; and FinnGen, which includes ICD diagnosis code-defined cases of MASLD.
Their study involved 66,814 individuals with hepatic steatosis defined by imaging and 3,584 cases (versus 621,081 controls) of MASLD defined by diagnosis codes.
“It was important for us to examine data in many individuals, as we now had the power to diminish the noise and ultimately observe new and significant genetic associations,” said Nicholette Palmer, Ph.D., co-senior author of the study from Wake Forest University School of Medicine.
“It was also important for us to use new neural network modeling to quantitate liver fat in more than 40,000 MRIs in the U.K. Biobank,” said Yanhua Chen, Ph.D., an application programmer and senior analyst at Michigan Medicine and first author of the study. “This provided us with high accuracy measurements in many MRIs where we previously did not have these measures. This increased our power to find reproducible hits.”
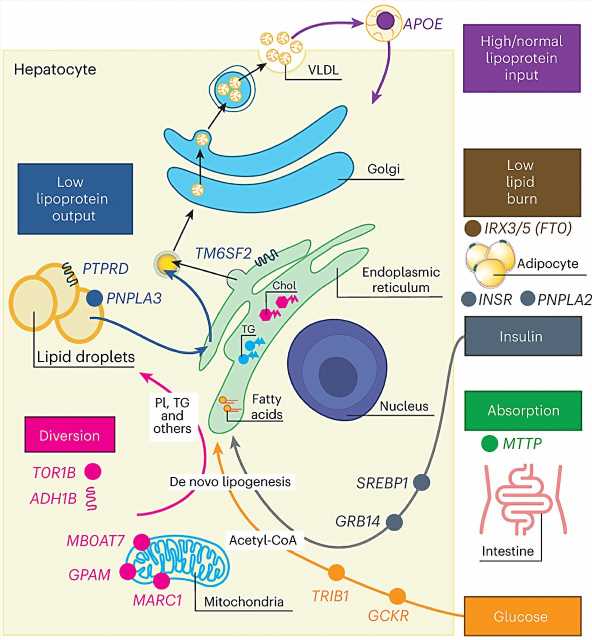
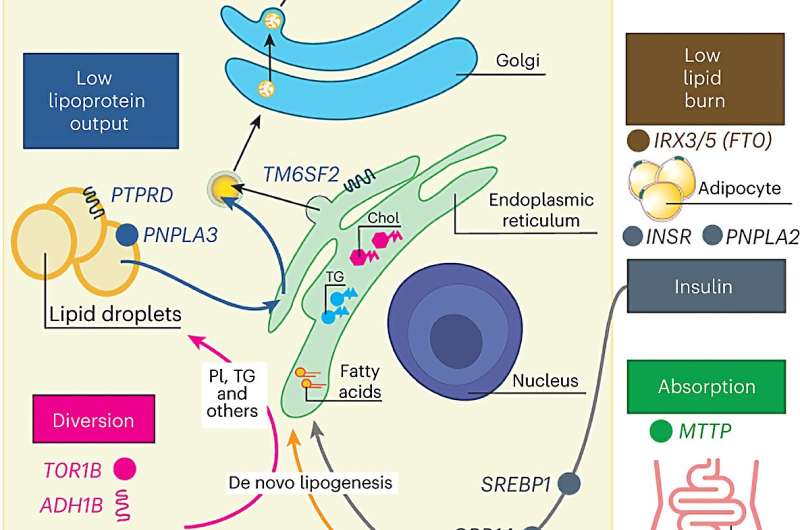
The team identified 17 genetic changes that contribute to MASLD, and by examining how these changes affected multiple human traits, the researchers were then able to identify seven subgroups of the disease.
According to Speliotes, these genetic markers can now be used as biomarkers to identify disease subtypes and link them to tissues, genes and pathways that can be targeted to treat them.
“Effective treatments in MASLD are greatly needed and we hope our findings will help guide the development and targeting of the right treatment, for the right patient, at the right time,” said Michigan Medicine’s Xiaomeng Du, M.S., co-first author of the study.
“Further, the genetic markers combined with each other can identify 10%, 5% and 1% of the population at two-, three- and four-fold increased risk of developing advanced liver diseases like cirrhosis and hepatocellular carcinoma,” Du continued.
Speliotes added that this is helpful because high-risk individuals are screened differently in hepatology when it comes to identifying and treating liver cancer early on, “when it is still curable.”
The team identified variation in liver lipid, cholesterol, steroid, alcohol and monocarboxylic acid processes as possibly disrupted in MASLD.
More specifically, they identified seven subclusters that affect lipid storage and release from hepatocytes, lipid recycling to the liver, peripheral adipose lipid storage and use, insulin signaling, intestinal absorption and glucose use (to make liver fat, break down fatty acids and/or enact the conversion of triglycerides and phospholipids.)
“Our research greatly increases our understanding of MASLD’s heterogenous causes,” said Speliotes.
She also said that she hopes the team’s findings will lead to better care for MASLD patients by identifying high-risk individuals before the disease develops.
“If the disease does develop, getting the right treatment at the right time maximizes the effectiveness of the available treatments with far fewer debilitating side effects,” she concluded.
More information:
Yanhua Chen et al, Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease, Nature Genetics (2023). DOI: 10.1038/s41588-023-01497-6
Journal information:
Nature Genetics
Source: Read Full Article