Simultaneous deep brain stimulation (DBS) and functional magnetic resonance imaging (fMRI) are critical for understanding the functional neural networks and the therapeutic effects and mechanisms of DBS therapies. Conventional DBS electrodes typically lead to severe distortion of the surrounding magnetic field, which creates large artifacts in MR images and thus impedes the functional investigation of DBS-fMRI.
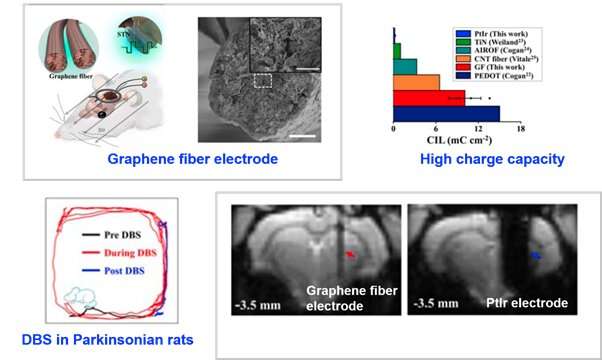
Recently, a collaboration between Dr. Duan Xiaojie’s group (Department of Biomedical Engineering, College of Engineering, Peking University) and Dr. Liang Zhifeng’s group (Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, CAS) has led to a novel MRI-compatible graphene fiber DBS electrode. Using a Parkinsonian rat model, this novel electrode achieved full activation pattern mapping by simultaneous deep brain stimulation and fMRI, and revealed a close relationship between fMRI activation and DBS therapeutic improvement.
This novel graphene fiber DBS electrode has 70 times more charge injection capacity than electrodes made of platinum-iridium (PtIr), which is the material most commonly used in clinical DBS, and exhibits much smaller artifacts in both T2-weighted structural images and T2-weighted functional echo planar images at a high field of 9.4-Tesla (Figure 1). In addition, this electrode shows high stability under continuous overcurrent pulsing. Using this graphene fiber DBS electrode, subthalamic nucleus (STN) was stimulated as the DBS target in a Parkinsonian rat model, which significantly improved rats’ motor ability and reduced the motor deficit.
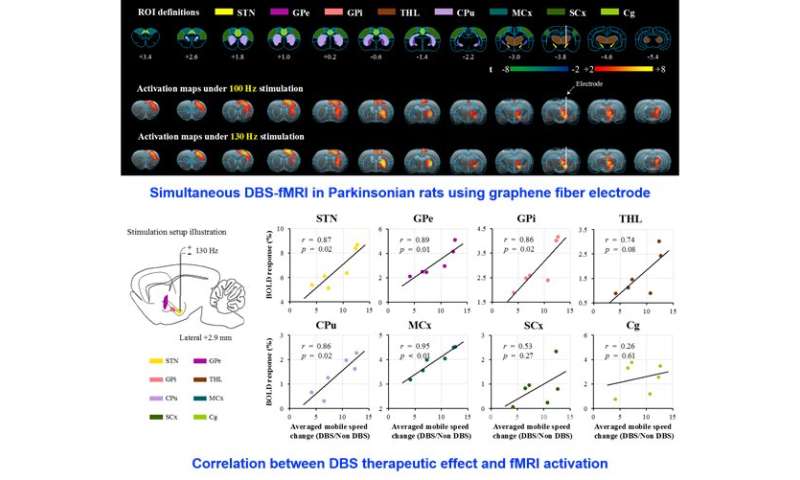
The unique advantage of this novel DBS electrode enabled full activation pattern mapping using simultaneous DBS and fMRI, including the DBS target (STN) itself. The fMRI activation amplitudes of several key brain regions were found to be closely associated with mobile speed improvement, including STN, motor cortex, internal globus pallidus (GPi), external globus pallidus (GPe) and caudate putamen (CPu) (Figure 2). The above result suggests that the therapeutic effect of STN DBS in Parkinson’s disease may be achieved by both orthodromic and antidromic effect of electrical stimulation.
Source: Read Full Article
