Most people with COVID-19 will experience a mild illness, and they’ll be able to take care of themselves at home. But some—especially those with underlying health conditions—could benefit from one of several COVID-19 treatments. Some of these are available in pill form and others are given intravenously or by injection—and all of them must be prescribed by a health care provider.
It’s important to remember that while new treatments are effective at reducing the severity of symptoms and helping prevent hospitalization and death in people who become infected with COVID-19, they are not a substitute for vaccination, which remains the single most effective strategy to prevent serious disease.
Below is Yale Medicine’s guide to COVID-19 treatments.
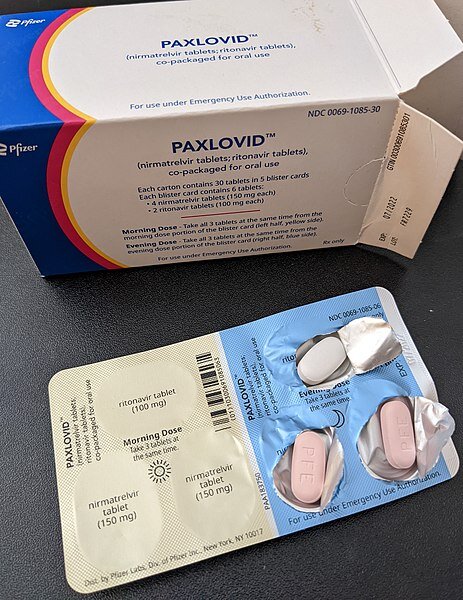
Paxlovid
What is it? Paxlovid is Pfizer’s brand name for an antiviral oral medication (in pill form) that combines two generic drugs, nirmatrelvir and ritonavir. It was the first COVID-19 antiviral pill to receive Food and Drug Administration (FDA) emergency use authorization (EUA), and the National Institutes of Health (NIH) has prioritized its use over other treatments for eligible patients. It is meant for people who have a current COVID-19 infection.
When it was authorized: December 2021.
Who can get it: People ages 12 and up who weigh at least 88 pounds, who have a positive COVID-19 test result, have symptoms, and are at high risk for developing severe COVID-19.
How you take it: For most people, the dose is three pills twice daily for five days, and it must be started within five days of developing COVID-19 symptoms.
Side effects: They’re usually mild, and may include altered or impaired sense of taste, diarrhea, increased blood pressure, or muscle aches. Because Paxlovid is still being studied, it’s possible that all of the risks aren’t yet known.
How it works: Paxlovid is an antiviral medication, a type of drug that stops viruses from replicating inside the body’s cells. Two of the pills in the three-pill dose are nirmatrelvir, which prevents the SARS-CoV-2 virus from replicating. The other medication is ritonavir, which gives the first drug’s levels a boost by essentially shutting down its metabolism in the liver, so that nirmatrelvir levels remain high and can work longer to fight the infection.
How well it works: 89% efficacy against hospitalization and death in the clinical trial, which included unvaccinated patients. Though the trial was conducted before omicron became the predominant variant, Pfizer says that the treatment appears to work well against it. This is backed up by three laboratory-based studies (all of which involved Pfizer) that have not yet been published in peer-reviewed medical journals.
What else you should know: Paxlovid interacts with many medications, including common ones that are sold over the counter like St. John’s Wort, blood thinners, cholesterol medicines, and many more. In some cases, this can cause complications that are serious enough to justify not taking it. So, it’s important for doctors to have an up-to-date medication list, including over-the-counter medications and supplements; they may consider other treatments for some patients.
There is no experience treating pregnant women or breastfeeding mothers with Paxlovid. Women who are pregnant should discuss their options with their health care provider. It is also recommended that patients use effective barrier contraception or do not have sexual activity while taking Paxlovid.
Paxlovid is also not recommended for patients with severe liver or kidney disease and those with HIV who are not on treatment.
More information: FDA Paxlovid fact sheet for patients, parents, and caregivers.
Remdesivir
What is it? The antiviral treatment remdesivir, sold under the brand name Veklury, was the first COVID-19 therapy to get full FDA approval, and, so far, it’s still the only one. Although originally used in COVID-19 patients only after they were hospitalized, new data suggests it can be helpful in outpatients who become infected and who are at high risk for severe disease. It is meant for people who have a current COVID-19 infection.
When it was authorized: Full approval was granted in October 2020. (It was first authorized in May 2020 for critically ill patients who were being treated with oxygen for COVID-19.) The authorization was later expanded to include other groups, and it was authorized to treat non-hospitalized patients in January 2022.
Who can get it: Fully approved for children and adults who are at high risk for severe disease. Infants and children must be at least 28 days old, weigh over 6.5 pounds or more, and be either hospitalized or at high risk for severe illness.
How you take it: Via injection or IV and administered only in a health care setting by a health care professional. For outpatients, the treatment is a three-day course of infusions that must be initiated within seven days of symptom onset.
Side effects: Nausea is the most common side effect. Hypersensitivity, including infusion-related and anaphylactic reactions, has been observed following treatment. There is insufficient data on the safety of using remdesivir in pregnant women or women who are breastfeeding; patients should speak with their health care provider.
How it works: Administered intravenously to patients who are in the hospital or in an ambulatory setting, the drug inserts itself into new viral genes to block replication of the virus, shortening the time it takes seriously ill patients to recover. A number of experts believe that the drug may work best early in the course of an infection.
How well it works: 87% reduction in risk of hospitalization in non-hospitalized patients given a three-day course, according to a study published in the New England Journal of Medicine in December 2021.
What else you should know: For hospitalized patients, research in early 2020 showed that the therapy reduced length of stay (the number of days in the hospital) from 15 days to 12. However, questions have been raised about remdesivir’s trial results for hospitalized patients. In late 2021, the World Health Organization (WHO) recommended against remdesivir after releasing data that showed disappointing results. Still, many U.S. hospitals continue to provide this medication.
This is one of two NIH-preferred therapies (after Paxlovid) for COVID-19.
More information: Gilead remdesivir fact sheet for patients.
Bebtelovimab
What is it? Bebtelovimab is an FDA-authorized investigational monoclonal antibody treatment that was developed by Eli Lilly. Not all authorized monoclonal antibodies have worked against all of the SARS-CoV-2 variants. However, data showing bebtelovimab’s efficacy against omicron and its BA.2 subvariant prompted the FDA to authorize the drug through an EUA. It is meant for people who have a current COVID-19 infection.
When it was authorized: February 2022.
Who can get it: Adults and children ages 12 and up who weigh at least 88 pounds. They must have a positive COVID-19 test result and be at high risk for developing severe COVID-19.
How you take it: An intravenous injection is given for at least 30 seconds. Patients are observed by a health care provider for at least an hour after injection. Bebtelovimab must be given within seven days of symptom onset.
Side effects: There is limited information known about the safety and effectiveness of bebtelovimab for the treatment of mild-to-moderate COVID-19, according to the FDA fact sheet. The sheet also provides a list of potential side effects the FDA recommends reporting to a medical provider, and reports that allergic reactions can happen during and after injection. Because bebtelovimab is still being studied, it’s possible that all of the risks aren’t yet known.
How it works: It binds to the spike protein that causes COVID-19, similar to other monoclonal antibodies that have shown efficacy against hospitalization and death from the disease.
How well it works: The EUA for bebtelovimab was supported by clinical and nonclinical data that showed it has efficacy against omicron and its BA.2 subvariant. The clinical data was based on a Phase 2 trial that treated non-hospitalized patients with bebtelovimab alone or together with another drug called etesevimab. That study is available in a preprint, which has not yet been peer-reviewed.
What else you should know: There is limited experience treating pregnant women or breastfeeding mothers. So, those patients should discuss their options and specific situation with their health care provider.
The NIH considers this to be an alternative treatment, which should be used only when neither of the NIH-preferred therapies (Paxlovid and remdesivir) are available, feasible to use, or clinically appropriate.
More information: FDA bebtelovimab fact sheet for patients, parents, and caregivers.
Molnupiravir
What is it? Molnupiravir, also known by the brand name Lagevrio, was developed by Merck and Ridgeback Biotherapeutics. It was heralded as a potential game-changer when the companies announced their initial clinical trial results in 2021. But when the data was finalized, it showed the drug to have lower efficacy than originally reported. Its FDA authorization came after a close vote that took into account the lowered efficacy and safety profile. The Centers for Disease Control & Prevention (CDC) now recommends that this drug should be used when the above-mentioned treatments aren’t available.
When it was authorized: December 2021.
Who can get it: People ages 18 and up who are at high risk for hospitalization and death from COVID-19.
How you take it: Four capsules every 12 hours (for example, at 8 a.m. and 8 p.m.) for five days. It must be taken as soon as possible, within five days of symptom onset.
How it works: When the drug enters the bloodstream, it blocks the ability of the SARS-CoV-2 virus to replicate.
How well it works: 30% efficacy against hospitalization and death. Merck initially reported the efficacy as 50%, but later adjusted that figure. Some laboratory studies from Merck have shown that molnupiravir is effective against the omicron variant.
Side effects: Diarrhea, nausea, and dizziness are the most common side effects. You should stop taking the pills right away if you have an allergic reaction. Because molnupiravir is still being studied, it’s possible that all of the risks aren’t yet known.
What else you should know: Molnupiravir is not recommended during pregnancy, since it has not been studied in pregnant women and has shown potential harm in in vitro studies—therefore, the true risk for harm to an unborn baby is unknown.
Individuals who are able to become pregnant should use reliable birth control during treatment and for four days after their last dose. It is also not known if molnupiravir could affect sperm, so individuals who are taking molnupiravir and who are sexually active with partners who are able to become pregnant should use reliable birth control during treatment and for three months after the last dose. (Studies to understand the risk to sperm beyond three months are ongoing.)
The NIH considers this to be an alternative treatment, which should be used only when neither of the NIH-preferred therapies (Paxlovid and remdesivir) are available, feasible to use, or clinically appropriate.
More information: FDA molnupiravir fact sheet for patients and caregivers.
Evusheld
What is it? Evusheld is a monoclonal antibody, but different than the other medications listed above. It combines two drugs, tixagevimab and cilgavimab. It is not designed to treat COVID-19; rather, its purpose is to keep immunocompromised people who do not respond to vaccination from getting sick. Developed by AstraZeneca, it is the first long-acting antibody to receive an EUA for pre-exposure prevention of COVID-19.
When it was authorized: December 2021.
Who can take it: Anyone 12 years or older who weighs at least 88 pounds and is at risk for severe illness—or those who cannot receive COVID-19 vaccines. Anyone taking the medication should have neither an active COVID-19 infection nor been recently exposed to a close contact who is infected.
How you take it: A health care provider will give one dose of Evusheld in the buttocks in two separate injections (of tixagevimab and cilgavimab, respectively), one after the other, with repeat doses every six months, while SARS-CoV-2 remains in circulation. Patients will be monitored for an hour after each injection. In March, the dosage for Evusheld was doubled, so patients who received the two injections prior to the change in dosage recommendations should talk to their doctor about the need to repeat treatment.
Side effects: Any intramuscular injection can cause hypersensitivity, pain, bruising, soreness, swelling, possible bleeding, or infection at the injection site. Tell your health care provider if you experience any allergic reactions during and after an injection. Serious but uncommon cardiac adverse events have occurred in the clinical trial.
Contact your health care provider or get medical help right away if you have any symptoms of cardiac events, including pain, pressure, or discomfort in the chest, arms, neck, back, stomach, or jaw, as well as shortness of breath, feeling tired or weak (fatigue), feeling sick (nausea), or swelling in your ankles or lower legs. Because Evusheld is still being studied, it’s possible that all of the risks aren’t yet known.
How it works: It combines two antibodies with different—and complementary—activities against the SARS-CoV-2 virus.
How well it works: According to a clinical trial, there was a 77% reduction in chances of getting COVID-19 initially; 83% six months after the treatment, according to the FDA news release. AstraZeneca says the drug should be effective for a year. It’s important to note that the exact efficacy against the latest variants is still unclear. (Note: Because the trial did not include immunocompromised patients, it is also unclear If the 77% reduction would apply to those who are immunosuppressed.)
What else you should know: Evusheld is intended as an additional benefit for those who may not respond to vaccination or who cannot be vaccinated. People who get Evusheld may need to receive additional doses for ongoing protection if new variants emerge. The best timing for additional doses, if needed, is not yet known; it will depend on which SARS-CoV-2 variant is in circulation.
Source: Read Full Article