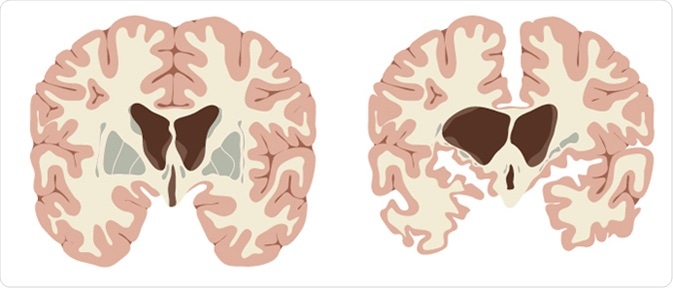
Huntington’s disease is a genetically inherited neurodegenerative disorder caused by an autosomal dominant mutation in the huntingtin gene resulting in a trinucleotide (CAG) repeat expansion with over 40 repeats.
Symptoms
Symptoms start with subtle cognitive changes, such as personality changes and altered mental ability. The patient then moves on to develop the more noticeable physical symptoms such as chorea (jerky and often random uncontrollable movements), lack of coordination, and abnormal posture and gait.
This eventually leads to abnormal facial expressions, dysphagia (swallowing difficulties), dysarthria (speaking difficulties), weight loss, dementia (cognitive decline), aggression, depression, compulsive behaviour (lack of inhibition) and insomnia.
Currently there is no cure for Huntington’s disease and it is typically fatal in 15-20 years after the initial symptoms. Full-time care is often needed for late-stage patients. To date, tetrabenazine is the only drug which can improve the quality of life by ameliorating some of the physical symptoms; however, it does increase the risk of severe depression in patients.
What are antibodies?
Antibodies are large Y-shaped proteins synthesized by the body in response to a pathogen. The antibody-producing cells then recognize any future attacks by the same pathogen. This phenomenon is also the basis of active immunization through vaccination.
Each antibody is highly specific to one antigen, which essentially tags one or more pathogens with this antigen so that the immune system can respond to the attack. This reaction may lead to macrophage recruitment to engulf the pathogens.
Highly specific antibodies may also be produced against any protein, including endogenous receptors and proteins, and this can cause specific therapeutic targeting.
Can antibodies be used to treat Huntington’s?
Semaphorin-4D (SEMA4D) is a protein that is involved in many processes that ultimately lead to inflammation in Huntington’s disease, among other neurodegenerative diseases. A humanised monoclonal antibody against SEMA4D is currently being developed to block or inhibit the action of SEMA4D to prevent neuroinflammation in disease pathogenesis.
Phase II clinical trials are going on, and are expected to be completed in 2020. Initial results do suggest that the antibody is safe and can be largely effective in ameliorating some key pathological changes associated with Huntington’s disease. Furthermore, in 2016 the FDA has approved a fast-track designation for this vaccine in the treatment of Huntington’s disease.
In addition, single-chain Fv antibodies (a fusion protein composed of different immunoglobulin components) have also been shown to bind to distinctive regions of the Huntingtin protein product, reducing protein aggregation and reducing cytotoxicity in tissue culture studies of Huntington’s disease. Although further translational work needs to be done using these types of antibodies, it is an encouraging therapeutic avenue.
Summary
Antibody-mediated treatments strategies in neurodegenerative diseases including Huntington’s disease promise to successfully slow disease progression by reducing neuroinflammation, as well as potentially restoring brain volume by actively encouraging brain recovery.
Current clinical trials and pre-clinical animal research is on to test various antibodies in the treatment of Huntington’s. Some of those trials will be completed in the next couple of years and their results will be important in changing the direction of neurodegenerative treatments, if proven to be successful.
Sources
- https://www.nhs.uk/conditions/huntingtons-disease/
- Abdulrahman GO, 2011. Yale J Biol Med 84(3):311-19 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178862/
- www.vaccinex.com/…/
- Rodrigues & Wild, 2018. J Huntingtons Dis. 7(3):279-86 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6087448/
Further Reading
- All Huntington's Disease Content
- What is Huntington’s Disease?
- Huntington’s Disease Symptoms
- Huntington’s Disease Diagnosis
- Huntington’s Disease Genetics
Last Updated: Jan 23, 2019

Written by
Osman Shabir
Osman is a Neuroscience PhD Research Student at the University of Sheffield studying the impact of cardiovascular disease and Alzheimer's disease on neurovascular coupling using pre-clinical models and neuroimaging techniques.
Source: Read Full Article
