Water, organic molecules, and inorganic ions are the constituents of cells. Water constitutes the greatest fraction of the three, accounting for almost three-quarters of the total mass of a cell. The interactions between the various components of a cell and its water content is key to biological chemistry.
Sodium, potassium, magnesium, calcium, phosphate, and chloride, are among the chief inorganic ions of a cell, and represent not more than 1% of the cell mass. But the organic molecules are the really novel components of a cell. Most such organic compounds belong to one of the following molecule classes:
- Carbohydrates
- Lipids
- Nucleic acids
- Proteins
Within individual cells, there exist thousands of different types of macromolecules, or organic compounds. These will be different, even among the cells of the same person. The variations are more extensive among different people. Macromolecules – proteins, nucleic acids, and polysaccharides – are formed by the polymerization of hundreds of their low-molecular-weight precursors – amino acids, nucleotides, and simple sugars.
The diversity among macromolecules evolves from the vast potential to form different combinations of the 50 or so common monomers which make up a macromolecule. These macromolecules may constitute up to 90% of a cell’s dry weight. It is possible to comprehend the basic chemistry of a cell’s makeup by understanding the functions and structures of the four major types of organic compounds, or macromolecules.
Carbohydrates
Carbohydrates are the basic building materials and nutrients of the body. Simple sugars and polysaccharides compose this group. Glucose is an example of a simple sugar that is an important cellular nutrient. Decomposition of the simple sugars by chemical reaction generates cellular energy as well as initiating the synthesis of other constituents of a cell. The polysaccharides, or complex carbohydrates, represent the form that sugar takes when it is stored. Polysaccharides are the structural components of a cell. Moreover, polysaccharides and other sugars may function as markers for certain cellular recognition processes, including the intracellular movement of proteins.
Lipids
Lipids are hydrophobic molecules. They are a highly efficient form of energy storage, and are major constituents of the cell membrane. They are important in cell signaling, function as the starting point for various biosynthetic processes such as the synthesis of estrogen and testosterone. Some lipids are able to convey signals from cell surface receptors to targets in the same or other cells. Phospholipids contain two fatty acids joined to a polar head group. Besides the phospholipids, cells have glycolipids and cholesterol.
Nucleic acids
Nucleic acids store and transmit hereditary data. DNA and RNA represent the informational molecules of a cell. DNA plays a crucial role as the genetic material of humans and many other species. RNA takes part in various cellular activities. Messenger RNA (mRNA) transports information from DNA to the ribosomes, where they are involved in synthesizing proteins. In addition, ribosomal RNA and transfer RNA are involved in protein synthesis. Other RNA molecules process and move both proteins and RNA. RNA can also catalyze chemical reactions, such as those involving the synthesis of proteins and the processing of RNA.
Proteins
Proteins play an important role in most of the tasks that an organism performs. Proteins carry out the work of a cell, directed by the genetic information carried by the nucleic acids. A cell holds many thousands of proteins, which function as a cell’s structural elements, storing and transporting small molecules, transmitting data among cells, and defending the body against the onset of infections. But proteins also function as enzymes that accelerate most chemical reactions. In this manner, proteins guide most cellular activities.
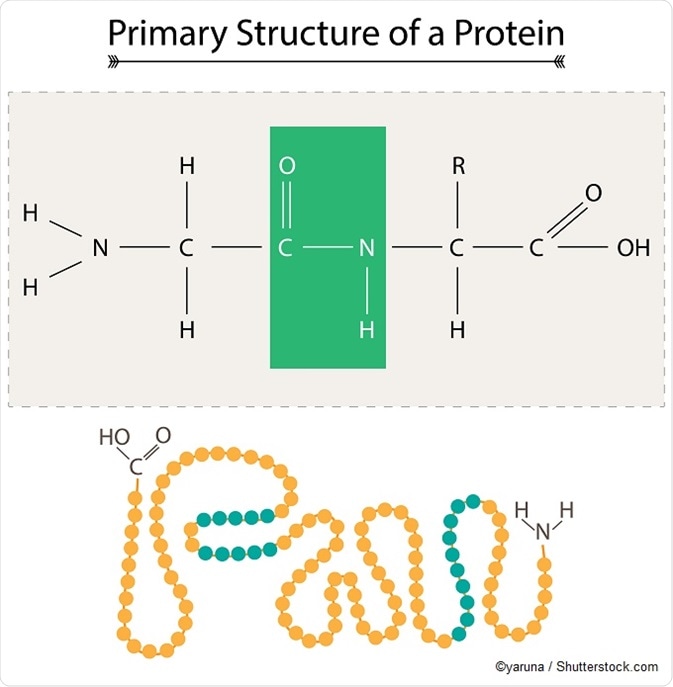
Structure and function
Covalent bonds, polarity, temperature, structure, and chemical reactivity are among the chemical factors which govern the structure and function of macromolecules. The structure of macromolecules determines how they function and regulate tasks. The 3-D structure of proteins and nucleic acids are controlled by non-covalent and covalent bonding, bestowing function on them. Meanwhile, it is possible to change the structure and function of proteins and nucleic acids by applying alternative splicing, alteration of the nucleotide sequence, or by chemical modification. Eventually, the structure and function of macromolecules can change over time to create different biological activity.
In terms of function, macromolecules harness non-covalent interactions when they inter-react with other molecules. Most biological functionality depends on the specificity and affinity of such interactions. The structure of macromolecules varies and changes over time. This is very important for biological functionality. It may be possible for small molecules to reach the interior of a macromolecule. The structure of macromolecules can influence the stable equilibrium of biochemical and molecular biological processes.
Additional resources
- Launch of next-generation Optima AUC advances protein research and macromolecule characterization
- A new method for manipulating macromolecules
- Researchers use large-scale computer modeling to show effects of confinement on cell macromolecules
- Scientists use PCT technique to understand physical effects of compression on macromolecules
- Transparent gel could soon become the first and best choice for sealing corneal incisions
References
- https://www.ncbi.nlm.nih.gov/books/NBK9879/
- https://www.asbmb.org/education/teachingstrategies/foundationalconcepts/MacromolecularStructureFunction/
- http://www.course-notes.org/biology/outlines/chapter_5_the_structure_and_function_of_macromolecules
Further Reading
- All Lipids Content
- What are Lipids?
- Lipid Biological Functions
- Lipid Metabolism
- Lipid Health and Nutrition
Last Updated: Aug 23, 2018

Written by
Joseph Constance
Joseph Constance has written about research, development, and markets in the health care and related fields. He has authored a number of articles, and business analysis/market research reports in the medical device, clinical diagnostics, and pharmaceutical areas. Joseph holds an MA from New York University in Communications. He enjoys spending time with his wife, biking, traveling, and learning about different cultures.
Source: Read Full Article
