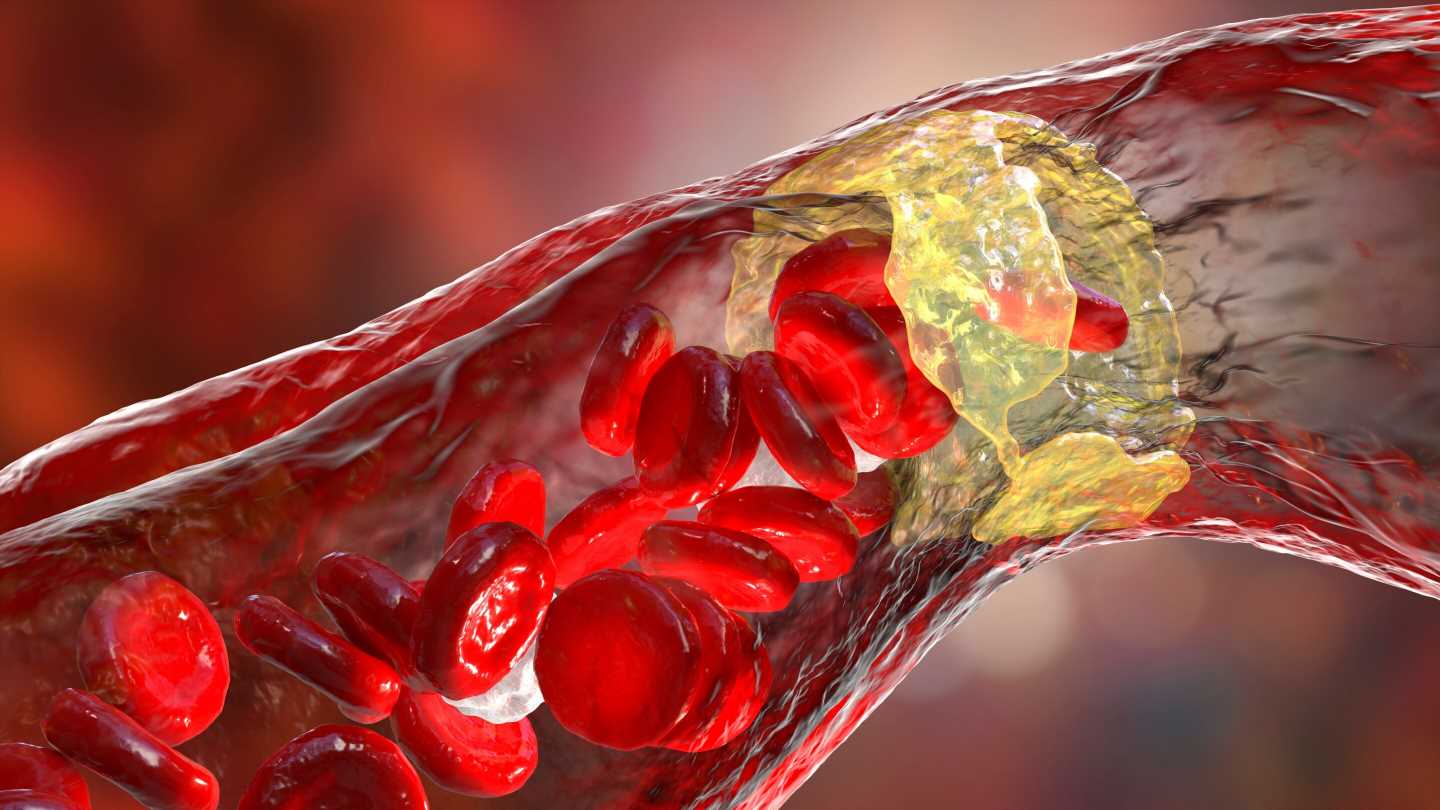
Heart attacks and strokes arising from atherosclerosis, which is driven by the accumulation of lipids in the inner linings of arteries, are responsible for about one third of deaths worldwide. Accordingly, atherosclerosis is the focus of intense research aimed at the development of novel effective treatments.
A team of researchers from MedUni Vienna has now described a key molecular pathway that provides new fundamental insights into the understanding of atherosclerosis progression and may pave the way for new treatment approaches.
Recent technological advancements have led to the discovery of a rich immune cell diversity in atherosclerotic lesions. In their study published July 26 in the journal Immunity, the team identified a specific subtype of complement-producing macrophages that are present in both mouse as well as human atherosclerotic plaques.
Regulation of cell-associated complement activity by complement factor H (CFH) limits the ability of these macrophages to clear dying cells, thereby aggravating plaque necrosis. Overwhelming necrotic cell death can lead to the instability of atherosclerotic plaques and result in subsequent cardiovascular events, such as heart attacks.
Complications of atherosclerosis, such as myocardial infarction and stroke are the leading causes of death worldwide. Last year, 34.7% of total deaths were caused by cardiovascular disease in Austria. Atherosclerosis is a disease of the arteries that is triggered by excessive lipid buildup, which promotes chronic inflammation in the vascular wall.
Atherosclerotic lesions are characterized by a profound infiltration of immune cells, including monocytes (the precursor cells of macrophages) and the concurrent accumulation of dying cells which can undergo necrosis if not efficiently handled.
The complement system is a family of blood-borne proteins with crucial importance in host defense from pathogens. In addition, complement exerts critical housekeeping functions by aiding the removal of damaged and dying cells by professional macrophages. Part of the complement is continuously active by the generation of cleavage products of the central component C3—a process that is highly regulated by CFH.
Complement activation has long been implicated in human atherosclerosis—however, the pathologic importance of cellular versus systemic complement activation in lesion progression has not been appreciated.
“In contrast to the conventional understanding that the role of complement in atherosclerosis is primarily driven by liver-derived complement via the circulation, there has been increasing evidence that immune cells can also produce a defined set of complement components. However, if and how complement is controlled within these cells has been unknown,” says Christoph J. Binder (Laboratory Medicine, MedUni Vienna), senior author of the study.
“We were able to demonstrate that inflammatory monocyte-derived macrophages accumulate complement C3, the central complement component during inflammation with a concomitant increase in the production of its master regulator, CFH.”
Functional CFH is critical to prevent excessive activation of the complement system in the circulation. Using numerous unique mouse models, the researchers dissected the role of CFH in atherosclerotic lesion progression.
“First, we made the surprising observation that global lack of CFH displays an overall beneficial impact on plaque progression, which is dependent on its interaction with C3. Building on these data, we were able pinpoint, that the protective effect is exerted on the cellular level, as selective deletion of CFH in monocytes and macrophages led to a robust decrease in both atherosclerotic lesion size and necrotic area due to an improved capacity to ingest and clear dying cells,” says lead author Máté G. Kiss.
“This is the first description that highlights the functional importance of a complement regulator to control complement activation within cells with a robust impact on the development of atherosclerosis, and potentially, other chronic inflammatory diseases, too.”
Their preclinical findings are matched with data providing clear implications to human cardiovascular disease. “Importantly, we identified a distinct inflammatory macrophage subset in human coronary artery plaques that is specifically enriched for C3 and CFH. Based on their gene expression profile, these cells are wired to respond to inflammation and appear to be critical for the engulfment of dying cells in human plaques—nevertheless, further investigation is warranted to test their functionality,” adds Binder.
Genetic variants in the CFH gene strongly predispose to chronic inflammatory diseases in humans. The same team has been carrying out groundbreaking research on identifying key genetic modulators of CFH in recognizing damaged cells and regulating complement activation.
“We could distinguish genetic variants that modify the ability of CFH to bind damaged macromolecules and dying cells. The same genetic variants may also alter the ability of CFH to regulate intracellular C3 levels in macrophages with an impact on their engulfing capacity,” notes Nikolina Papac- Miličević, co-first author of the study.
More information:
Máté G. Kiss et al, Cell-autonomous regulation of complement C3 by factor H limits macrophage efferocytosis and exacerbates atherosclerosis, Immunity (2023). DOI: 10.1016/j.immuni.2023.06.026
Journal information:
Immunity
Source: Read Full Article