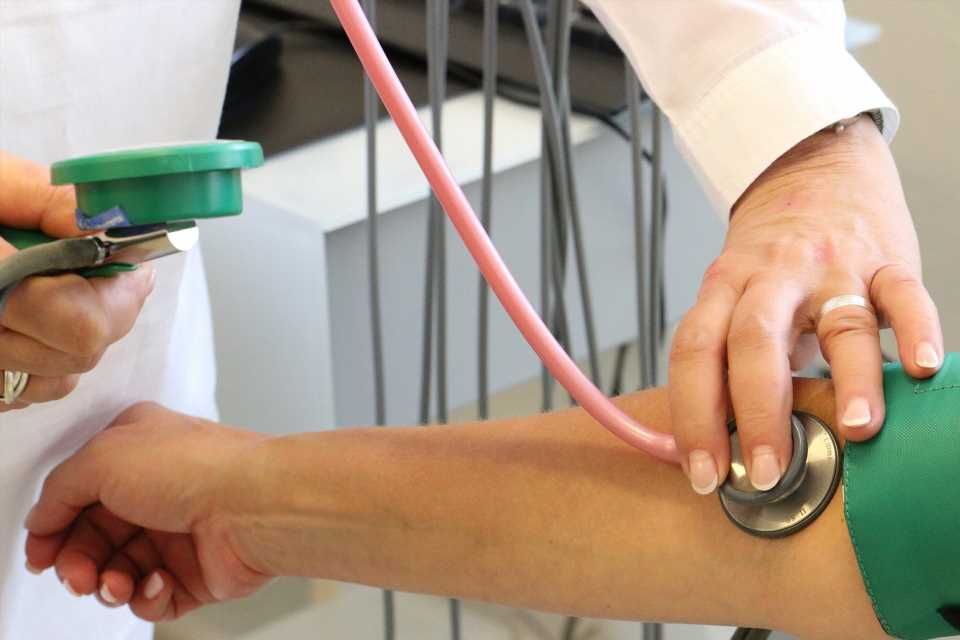
A pragmatic randomized trial in more than 21,000 patients with high blood pressure followed for over five years has concluded that protection against heart attack, stroke and vascular death is not affected by whether antihypertensive medications are taken in the morning or evening. The late breaking research is presented in a Hot Line session today at ESC Congress 2022 and contradicts previous findings that suggested a very large cardiovascular benefit of night-time dosing.
More than one billion people have high blood pressure worldwide. High blood pressure is the leading global cause of premature death, accounting for almost ten million deaths in 2015, of which 4.9 million were due to ischemic heart disease and 3.5 million were due to stroke. Nocturnal blood pressure is a better predictor of cardiovascular outcomes than daytime blood pressure, and there is previous evidence that antihypertensive drugs taken in the evening rather than in the morning reduced night-time blood pressure to a greater extent. The Hygia study previously indicated a protective effect of nocturnal dosing on cardiovascular events, but this study has attracted criticism.
TIME was a large prospective, randomized trial conducted to test whether evening dosing of antihypertensive medication improved major cardiovascular outcomes compared with morning dosing. Adults taking at least one antihypertensive medication and with a valid email address were recruited by advertising in the community, from primary and secondary care, and from databases of consented patients in the UK.
After participants had signed up on the TIME website and their eligibility was confirmed, they were randomized 1:1 to take their usual antihypertensive medication in the morning or the evening. The composite primary endpoint was hospitalization for nonfatal myocardial infarction or nonfatal stroke, or vascular death, in the intention-to-treat population. Information on hospitalizations and deaths was obtained from participants by email and through record linkage to national databases and further data was gathered from family doctors and hospitals and independently adjudicated by a committee blinded to allocated dosing time.
A total of 21,104 patients were randomized, 10,503 to evening dosing and 10,601 to morning dosing. The average age of participants was 65 years, 58% were men and 98% were white. The median follow-up duration was 5.2 years but some were in the study for over nine years. The primary endpoint occurred in 362 (3.4%) participants in the evening dosing group (0.69 events per 100 patient years) and 390 (3.7%) in the morning dosing group (0.72 events per 100 patient years), giving an unadjusted hazard ratio of 0.95 (95% confidence interval 0.83–1.10; p=0.53). The results did not vary in pre-specified subgroup analyses. Taking medication in the evening was not harmful.
Source: Read Full Article