Unlike other infectious diseases that affect the lungs, the immune response to fight tuberculosis (TB) infections develops at least twice as slowly. Until recently, the dynamic interplay between bacteria and the host’s immune system remained unclear, hampering the development of effective therapies against the disease, which kills more people worldwide than HIV/AIDS and is second only to COVID-19.
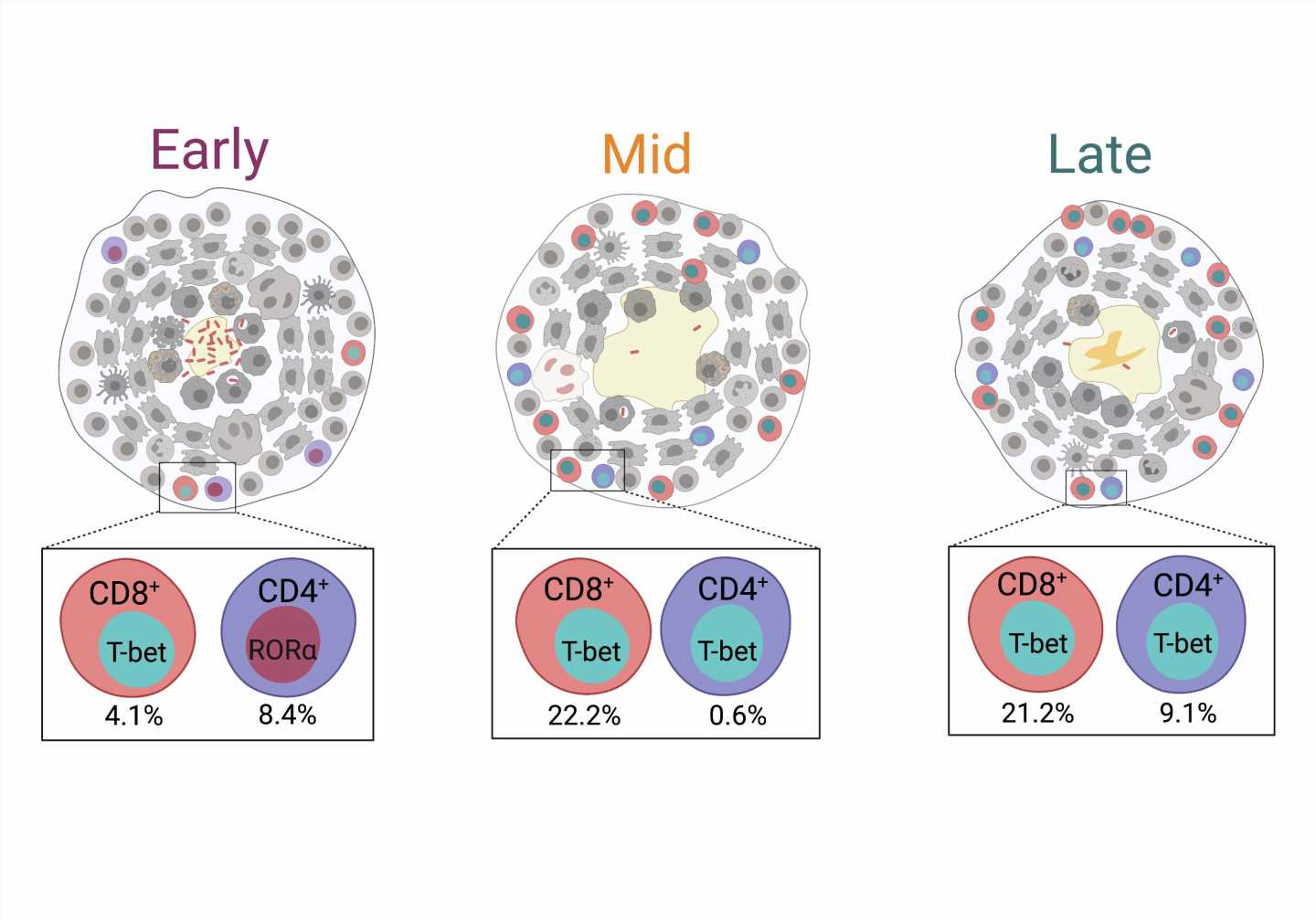
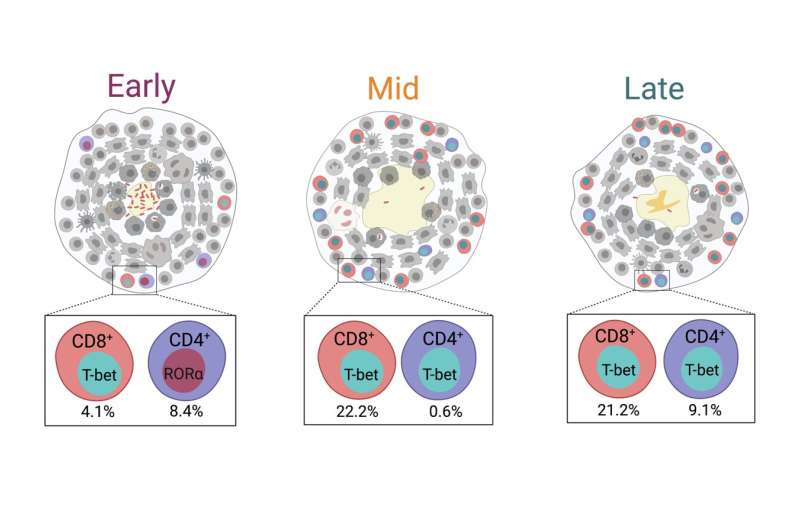
In a paper published in Cell Reports today, University of Pittsburgh School of Medicine immunologists discovered that adaptive immune response against Mycobacterium tuberculosis—pathogenic bacteria that cause TB—matures over time. The first subset of infection-fighting T lymphocytes does not become fully active until three months after the infection, and the emergence of a second subset of T cells at five months post-infection can contribute to bacterial clearance and recovery.
Such delayed adaptive immune response partially explains why TB infections easily take hold in the host’s lungs and suggests that vaccination strategies against TB should be adjusted to prime T cell responses and kick them into gear at the early stages of infection, ensuring that pathogenic bacteria can be eliminated quickly.
“Lung TB infections can start with a single bacterium that divides into hundreds of thousands of bacteria over the course of four to six weeks. Not a lot of bacterial killing happens between then and 10 weeks post-infection,” said senior author JoAnne Flynn, Ph.D., distinguished professor of microbiology and molecular genetics at the University of Pittsburgh. “In our study, we wanted to find out what changes in the lung immune environment over time that allows it to eventually bring TB infection under control.”
While TB is relatively uncommon in the U.S., it remains a major concern in many countries around the world, disproportionately affecting people in developing countries.
TB is a severe respiratory disease caused by bacteria that slowly propagate and expand upon entering the lungs through the airways, causing lung scarring and inflammation, severe cough and chest pain. To wall off the growing bacteria, the body responds by creating cellular barriers called granulomas, organized spheres of cell clusters made up of immune cells that are a tell-tale sign of TB.
And though most people without underlying health conditions can control TB infections and remain asymptomatic without medical assistance, those with weakened immune systems, such as people with HIV/AIDS or those sick with COVID-19, are at high risk of developing complications and succumbing to the disease.
The only widely used vaccine against TB, called the bacille Calmette-Guérin (BCG) vaccine, is administered to newborns within the first few hours of life. However, the BCG vaccine is limited in its effectiveness and does not prevent infections in the lungs.
Flynn’s previous work showed that administering the BCG vaccine intravenously, as opposed to traditional injection under the skin, offers nearly complete protection against TB infections in monkeys. The new study in Cell Reports provides insights into how this vaccine strategy might be working by speeding up the T cell responses in the lungs so they can quickly shut down infection.
By detecting TB granulomas in macaque monkeys’ lungs using a state-of-the-art imaging technique that allows them to scan lungs of live animals in real time and track development of granulomas as the infection progresses, the researchers were able to isolate granulomas at different times post-infection.
After carefully excising the granulomas and quantifying their cellular composition, researchers found that key players in adaptive immunity, CD8+ T cells, become activated by three months after infection and are joined by CD4+ T cells that further support infection clearance at five months post-infection.
The emergence of these activated T cells is inversely correlated with the number of granuloma-contained live bacteria, suggesting that these cells play critical roles in bacterial control.
“Our work highlighted the timing of evolving adaptive immune response and bacterial clearance in TB,” said first author Nicole Grant, Ph.D., postdoctoral fellow at Pitt. “Harnessing that knowledge to increase the number of functional T cells in the early days of infection and getting them to respond and contain bacterial growth quicker is key to creating a therapy that can shut down the infection at its onset.”
Source: Read Full Article