From chemotherapy drugs that affect the whole body to very precise surgery to remove a tumor, different cancers require different treatments.
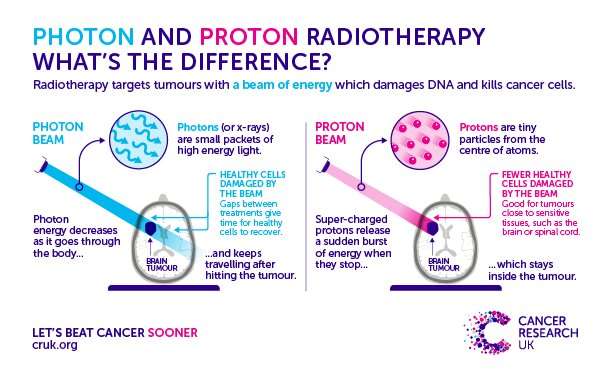
One treatment that has been used for over a century is radiotherapy, which involves the use of high energy photons—such as X-rays or gamma rays. Rather than subjecting the entire body to treatment, this beam is only aimed directly at the tumor and damages the cells’ DNA, causing them to die.
Despite being so specific, there’s still a chance that this treatment could damage the normal tissue surrounding the tumor—which is never good news. This can lead to a range of short- and long-term side effects (including fatigue, nausea, and memory loss), depending on where the tumor is located.
Enter proton beam therapy. This more recent and advanced form of radiotherapy uses protons (positive-charged particles) to target the tumor cells. These protons are sped up using a piece of equipment called a particle accelerator and fired as a beam at the tumor, stopping once they hit the cancer cells.
In principle, this means less damage to cells around the tumor and the potential to give stronger, more targeted treatment. But with this technology being so new, how can we be sure that it does less damage to healthy cells than conventional radiotherapy treatments?
This is something that a team of our researchers across the UK are hoping to find out with the first proton beam therapy clinical trial in the UK.
Putting it to the test
TORPEdO is a phase 3 trial taking place at The Christie NHS Foundation Trust in Manchester. It’s being led by cancer specialist Dr. David Thomson to test if this innovative new form of radiotherapy can improve throat cancer treatment.
“What we’re trying to do is work out if we can improve the long-term side effects and quality of life for patients who are treated with chemotherapy and radiotherapy,” says Thomson.
For 8 in 10 of the people with cancers of the throat (which includes cancers of the tonsils, base of the tongue and other parts of the oropharynx), radiotherapy is a key part of their treatment. Depending on their location, treating these cancers can be complicated by the many important organs sitting nearby.
Damage to the healthy tissue in the mouth and throat can have lasting impacts—from difficulties chewing, problems with dryness or swallow function to long-term hearing loss—and this is where the researchers think proton beam radiotherapy could help.
Funded by Cancer Research UK and The Taylor Family Foundation, the trial’s goal is to work out whether proton beam therapy reduces toxicity compared with conventional radiotherapy treatment for people with throat cancer (oropharyngeal squamous cell carcinoma).
And for Thomson, it’s the logical next step. “Currently the Proton Beam Therapy Unit at The Christie is mainly used for children and young adults,” says Thomson. “We would like to make the facility available for other groups of adult patients who may benefit most from it too. The first step is to determine whether proton beam therapy improves side effects and quality of life for patients with throat cancer.”
The trial kicked off in February 2020 and despite the pandemic, the team have made impressive progress. Although it was closed amid-2020 by the lockdown, Thomson says the proton beam therapy trial was one of the first non-COVID-19 studies to reopen. Three centers are now open and recruitment is running ahead of schedule, with 35 participants recruited. Over the next 18 months, a total of 20 centers will open to co-ordinate the nationwide trial.
A total of 183 adults with oropharyngeal squamous cell carcinoma will be randomized into two groups, with two thirds having proton beam therapy (at either The Christie in Manchester or at UCLH in London) and the rest receiving standard, intensity modulated radiotherapy.
Beverley Ashworth, a 52 year old care worker from Middleton in Greater Manchester, was first diagnosed last summer. Beverly is taking part in the trial and recently finished her treatment. “I had treatment for seven and a half weeks but it felt like no sooner had it started than it’d finished. I felt well for most of the time and I couldn’t have had nicer people looking after me.
Beverly said that initially she had to mainly stick to soup as she couldn’t swallow, but not she’s able to eat toast again and lots of spaghetti and beans. “I did have to have a feeding tube in for a couple of weeks but luckily that was taken out just before Christmas. I know the recovery will be slow but I’m getting there. I was very happy to participate in the trial and have already signed up for another one. If it wasn’t for clinical trials we wouldn’t find new treatments for patients in the future.”
Looking to the future
For the rest of the year, the researchers are focusing on increasing the number of people taking part in the trial and making sure that all the systems are in place for research data to be analysed. And there will be quite a lot of data—as well as questionnaires collecting information on people’s experiences during the trial, everyone taking part will be followed up for 5 years to find out if they’re experiencing any longer-term side effects.
But the team aren’t stopping there. They’re also asking participants if they want to take part in smaller studies linked to the trial—ensuring they’re getting as much information as possible from the trial. So far, 90% of trial participants have signed up to have their blood samples used to assess changes in cancer biomarkers—such as the immune response and tumor DNA levels—to help the team to explore how the treatments changes the biology of the tumor and surrounding cells.
Thomson thinks that the results the trial and its connected studies could have far-reaching effects in the world of personalized medicine. “It’s all part of the big picture about how we can actually marry up the clinical response with the change in biology and imaging. They’re all linked.
Source: Read Full Article
