In Alzheimer’s disease, the degeneration of brain cells is linked to formation of toxic protein aggregates and deposits known as amyloid plaques. Similar processes also play an important role in type 2 diabetes. A research team under the lead of the Technical University of Munich has now developed “mini-proteins,” so-called peptides, which are able to bind the proteins that form amyloids and prevent their aggregation into cytotoxic amyloids.
Many cell and neurodegenerative diseases are linked to the formation of toxic protein aggregates which cause cell death. Prominent representatives of these diseases are Alzheimer’s disease and type 2 diabetes mellitus, with worldwide more than 50 million and 400 million patients, respectively. Importantly, the number of Alzheimer’s and diabetes patients continues to rise, as the population becomes older. However, the two diseases remain so far incurable. Therefore, there is an urgent need for new therapeutic approaches.
Targeting the formation of harmful amyloid aggregates is a promising approach. A team led by Aphrodite Kapurniotu, a professor for Peptide Biochemistry at the Technical University of Munich (TUM), has now developed novel synthetic peptides, which are able in experimental models to block toxic amyloid aggregation linked to both diseases. Their research is published in Nature Communications.
Molecular interactions between Alzheimer’s disease and type 2 diabetes
Previous studies showed that certain “cross-interactions” between the amyloidogenic proteins of the two diseases dramatically accelerate their amyloid aggregation process. These findings could possibly explain why people suffering from one of the two diseases might have an increased risk for the other disease as well.
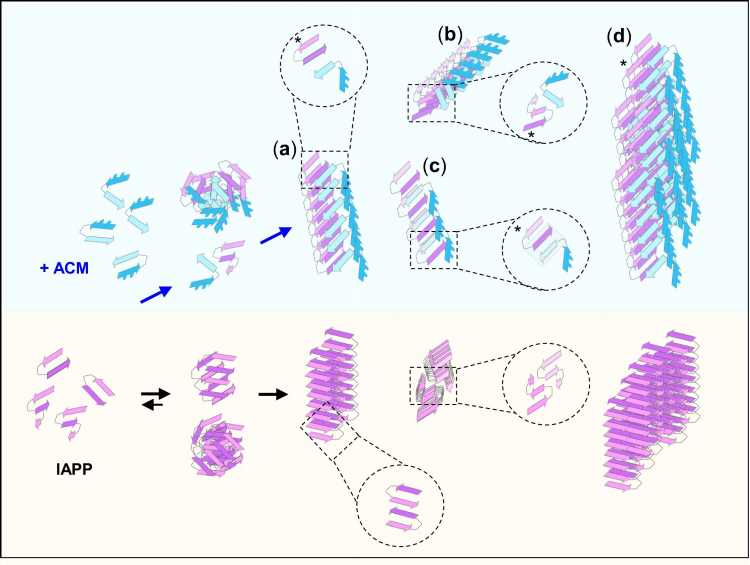
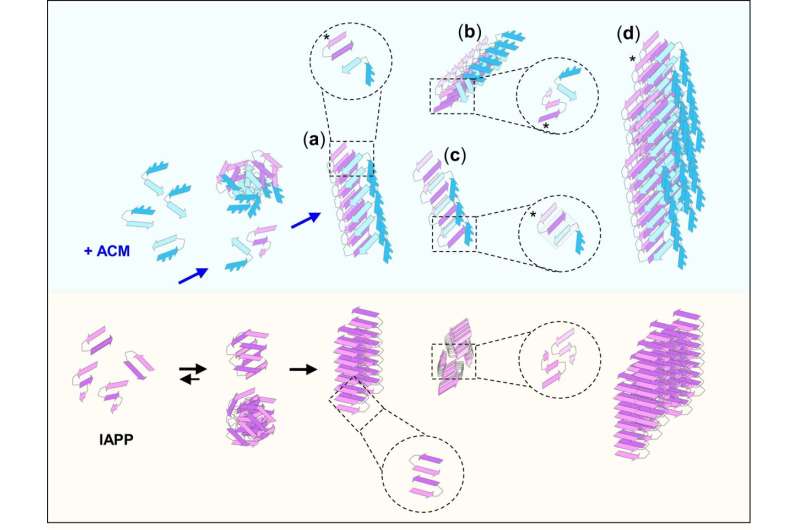
The team developed synthetic peptides that could function as effective inhibitors of amyloid aggregation in both diseases. Prof. Kapurniotu says, “The designed peptides are in fact able to bind the amyloidogenic proteins linked to both diseases and to effectively suppress both cytotoxic amyloid aggregation and amyloid cross-accelerating interactions. Remarkably, although the mixed aggregates formed by interactions of the designed peptides with the amyloidogenic proteins look very similar to harmful amyloid aggregates, they are completely devoid of cytotoxic effects. Moreover, these amyloid-resembling mixed aggregates become more efficiently taken up by the phagocytic immune cells than amyloid aggregates.”
Future studies to pave the way for medical application
Increasing evidence suggests that Alzheimer’s disease and type 2 diabetes are linked to each other. Prof. Kapurniotu believes thus that the designed peptides could be valuable candidates for the development of drugs for treating both diseases.
A patent application has been already filed by TUM. Additional studies are now planned to translate the findings from the experimental models into the clinic.
More information:
Karin Taş et al, Designed peptides as nanomolar cross-amyloid inhibitors acting via supramolecular nanofiber co-assembly, Nature Communications (2022). DOI: 10.1038/s41467-022-32688-0
Journal information:
Nature Communications
Source: Read Full Article