Early diagnosis and timely treatment are extremely important to manage dry eye disease (DED), a common condition exacerbated by the increased use of digital devices. But the current method of diagnosis is invasive, and its results are inconsistent with the subjective symptoms of dry eye disease. To solve this issue, a research team—led by researchers from Juntendo University Graduate School of Medicine—has developed DryEyeRhythm, as a non-invasive and reliable mobile app to assess DED.
Dry eye disease (DED) is a condition characterized by an array of symptoms, including dryness, ocular discomfort, fatigue and visual disturbances. This condition has become increasingly common in recent years owing to an aging society, increased screen time, and a highly stressful social environment. There are about 1 billion people, worldwide, who have DED. Undiagnosed and untreated DED can lead to a variety of symptoms, including ocular fatigue, sensitivity to light, lower vision quality, and a lower quality of life. Given the widespread prevalence of the condition, this can further lead to reduced work productivity and economic loss.
Despite the obvious disadvantages of DED, a large portion of the population remains undiagnosed, which ultimately leads to increased disease severity. DED is currently diagnosed through a series of questionnaires and ocular examinations (which can be invasive). But this method of diagnosis is not ideal. DED examinations do not always correspond with patients’ subjective DED symptoms. Furthermore, non-invasive and non-contact dry eye examinations are required in the COVID-19 pandemic. These flaws point to a need for a simple, reliable, and accessible screening method for DED to improve diagnosis and prognosis of the disease.
To answer this need, a research group, led by Professor Akira Murakami and Associate Professor Takenori Inomata of the Juntendo University Graduate School of Medicine, developed a smartphone application called DryEyeRhythm. “DryEyeRhythm leverages the cameras in smartphones to measure users’ blink characteristics and determine maximum blink interval (MBI)—a substitute for tear film breakup time, an important diagnostic criterion of DED,” explains Associate Prof. Inomata. “The app also administers Ocular Surface Disease Index (OSDI) questionnaires, which are also a crucial component of DED diagnosis.”
To validate the usefulness of the app, the research team conducted a prospective, cross-sectional, observational, single-center study, the results of which have been published in The Ocular Surface.
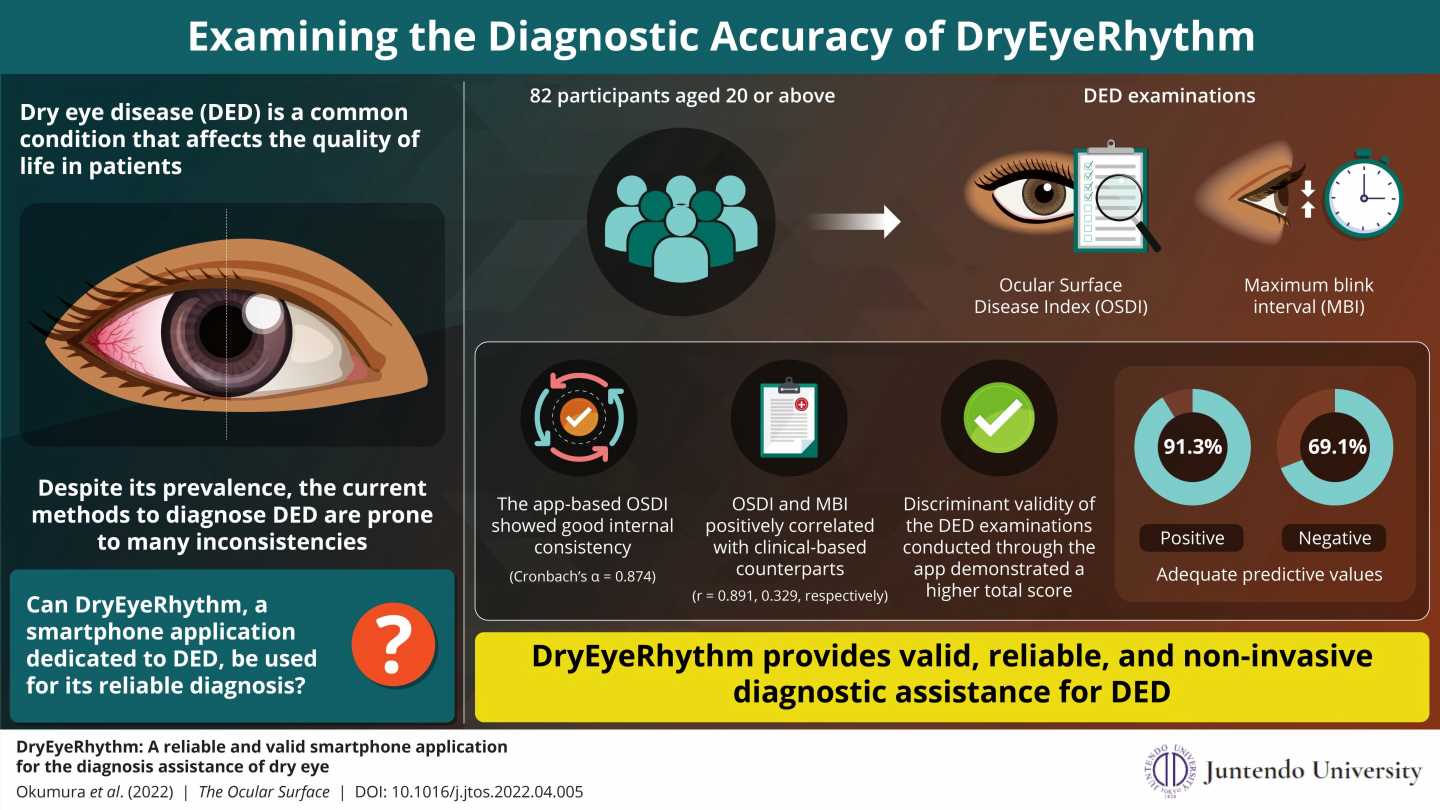
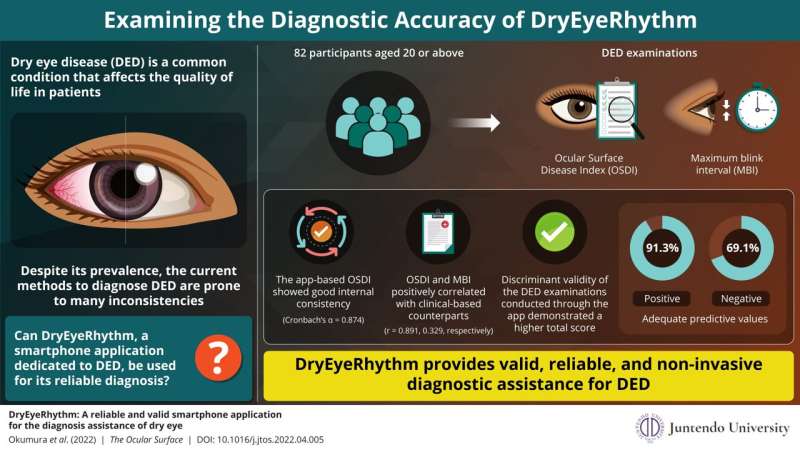
For their study, the team recruited 82 patients, aged 20 years or older, who visited the ophthalmology outpatient clinic at the Juntendo University Hospital between July 2020 and May 2021. The participants completed the Japanese version of the OSDI questionnaire (J-OSDI) and underwent examinations for MBI, both via the app and via other analysis techniques.
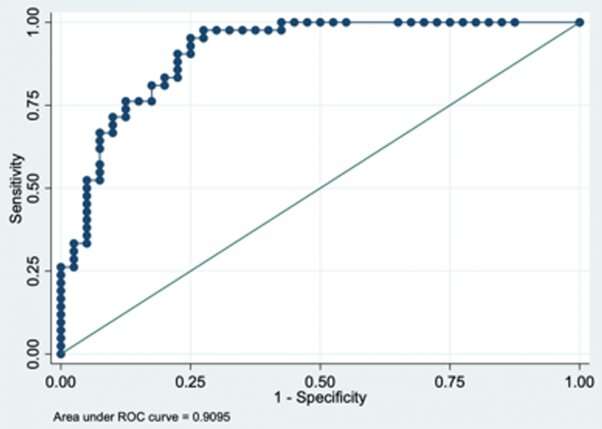
The study revealed that the J-OSDI collected with DryEyeRhythm showed good internal consistency. Moreover, the app-based questionnaire and MBI yielded significantly higher discriminant validity. The app also showed good positive and negative predictive values, with 91.3% and 69.1%, respectively. The area under the Receiver operating characteristic (ROC) curve—a measure of clinical sensitivity and specificity—for the concurrent use of the app-based J-OSDI and MBI was also high, with a value of 0.910. These results demonstrate that the app is a reliable, valid, and moreover non-invasive, instrument for assessing DED.
“Non-contact and non-invasive DED diagnostic assistance, like the kind provided by DryEyeRhythm, could help facilitate the early diagnosis and treatment of patients, as well as, DED treatment through telemedicine and online medical care,” says Associate Prof. Inomata. The research team plans to further validate its results by conducting a multi-institutional collaborative study in the future. They are also planning to obtain medical device approval and insurance reimbursement for the smartphone application.
Source: Read Full Article